water
You are only ever a few days away from your demise.
As droughts threaten water supplies across the planet, some municipalities aim to utilize an untapped resource: sewage water.
Scientists at Washington University are patenting a new electrolyzer designed for frigid Martian water.
A clever new design introduces a way to image the vast ocean floor.
Scientists discover that under certain conditions two kinds of water exist.
An ancient Martian meteorite carries with it some compelling implications.
A new study from NASA and the SETI Institute comes up with an exciting number of potentially life-supporting planets.
A new study finds the rocks that first formed Earth carried with them enough hydrogen for three times the water we have today.
A new study examines the under-researched area of water theft around the world.
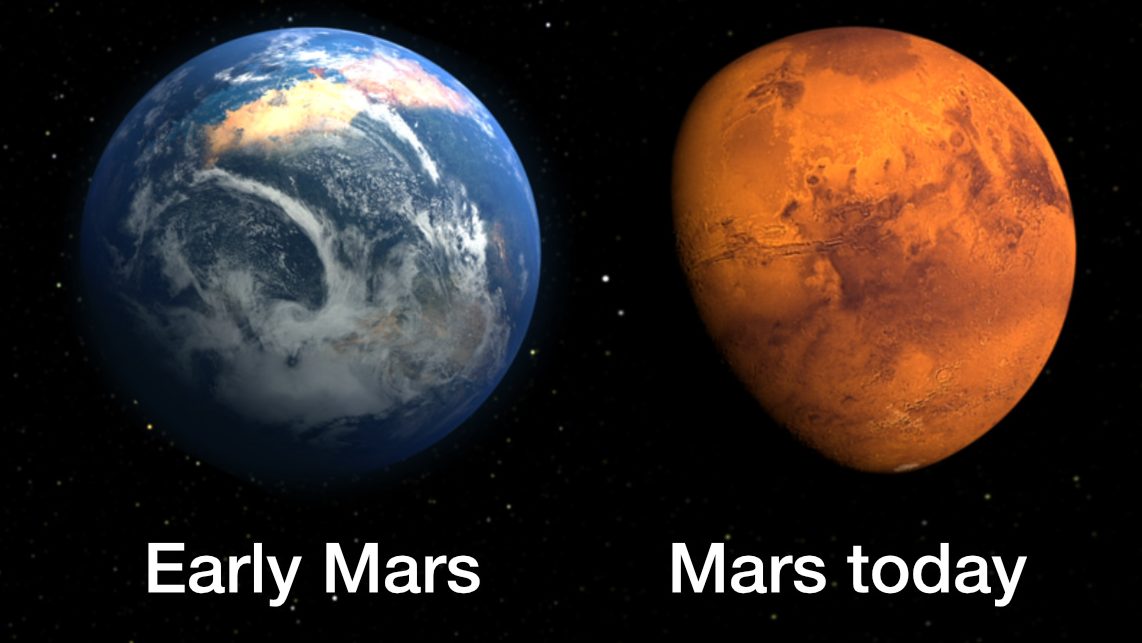
A 71% wet Mars would have two major land masses and one giant ‘Medimartian Sea.’
The ocean’s largest shark relies on vision more than previously believed.
Researchers devise an effective new predictive tool for maritime first-responders.
A fun and completely safe experiment for the family to try during quarantine.
▸
5 min
—
with
An open letter predicts that a massive wall of rock is about to plunge into Barry Arm Fjord in Alaska.
Researchers think they know how a group of ancient sloths, who died thousands of years ago in Ecuador, met their untimely end.
Non-avian dinosaurs were thought terrestrially bound, but newly unearthed fossils suggest they conquered prehistoric waters, too.
Coke, meth, ecstasy, amphetamines: each drug has a different ‘capital’
A new study says that it could be centuries before millions of the classic toys submerged in the Earth’s seas disintegrate.
The Hollywood blockbuster may have been right, if only 3.2 billion years off the mark.
The origin and phylogeny of the Yaravirus are not yet clear.
New research suggests the ocean current that delivers warm water to Europe has a one-in-six chance of halting temporarily over the next hundred years, potentially resulting in freezing temperatures.
You’ve likely heard of solar energy, but what is osmotic energy?
Thinning forests in the Western United States can save billions of gallons of water per year and improve conservation efforts.
After China stopped accepting recylables, California was put in a tough place.
Clusters of bot boats may offer cities dynamic solutions to rising waters.
Researchers from UCLA invent a device that generates electricity from a rather unusual source.
Turning salt water into fresh water with the power of the sun.
A loophole signed into law during the Bush administration has been fiendishly tough to close.
A new research letter points to another reason for childhood obesity.