This ‘brine electrolyzer’ can mine oxygen, hydrogen from water on Mars

Credit: NASA/JPL-Caltech
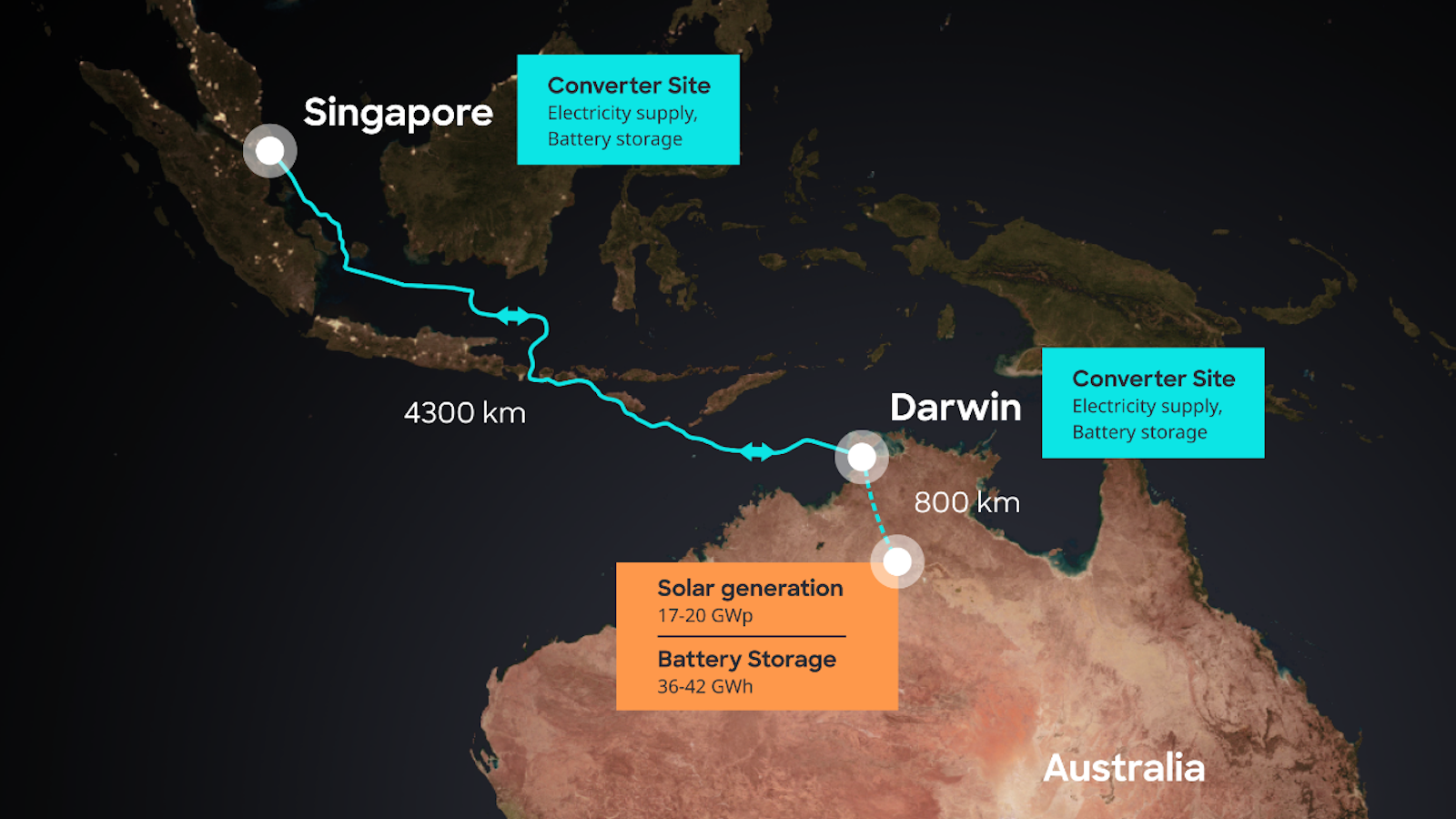
- Mars explorers will need more oxygen and hydrogen than they can carry to the Red Planet.
- Martian water may be able to provide these elements, but it is extremely salty water.
- The new method can pull oxygen and hydrogen for breathing and fuel from Martian brine.
When people finally get to Mars, there are a two things they’re going to need lots of: oxygen and fuel. (Drinking water, too, but that’s another story.) They’ll need more than they could reasonably bring with them. Fortunately—we now know—there’s plenty of water on Mars that could potentially serve as a source of oxygen and of fuel in the form of hydrogen that could get our explorers back home at mission’s end.
On Earth, we can extract the elements from pure water using a process called electrolysis. On Mars, though, the water contains a fair amount of magnesium perchlorate — salt. It is too “dirty” for electrolysis, and that process also requires heat, an issue in Mars’ frigid climate. Engineers at Washington University (WashU) in St. Louis may have the solution. They’ve developed and are in the process of patenting a method for extracting oxygen and hydrogen from water like Martian brine, and it works perfectly well in sub-zero temperatures.
“Our Martian brine electrolyzer radically changes the logistical calculus of missions to Mars and beyond,” says WashU’s Vijay Ramani.
The system is described in a paper published in PNAS.
The WashU electrolyzer—it has no snappy acronym yet—will not be the first device capable of extracting oxygen from Martian water. That honor goes to the Mars Oxygen In-Situ Resource Utilization Experiment, or MOXIE, which is en route to Mars onboard NASA’s Perseverance rover. The rover was launched on July 30, 2020. It will arrive on February 18, 2021, and will perform high-temperature electrolysis to extract pure oxygen, but no hydrogen.
In addition to being able to capture hydrogen, the WashU system can even do a better job with oxygen than MOXIE can, extracting 25 times as much from the same amount of water.
The new system has no problem with Mars’ magnesium perchlorate-laced water. On the contrary, the researchers say it ultimately makes their system work better since such high concentrations of salt keep water from freezing on such a cold a planet by lowering the liquid’s freezing temperature to -60 °C. He adds it may “also improve the performance of the electrolyzer system by lowering the electrical resistance.”
Cold itself is no issue for the WashU system. It’s been tested in a sub-zero (-33 ⁰F, or -36 ⁰C) environment that simulates Mars’.
“Our novel brine electrolyzer incorporates a lead ruthenate pyrochlore anode developed by our team in conjunction with a platinum on carbon cathode,” explains Ramani. He adds, “These carefully designed components coupled with the optimal use of traditional electrochemical engineering principles has yielded this high performance.”
“This technology is equally useful on Earth where it opens up the oceans as a viable oxygen and fuel source,” Ramani notes. His colleagues forsee potential applications such as producing oxygen in deep-sea habitats with ample water available, such as underwater research facilities and submarines.
The study’s joint first author Pralay Gayen says that “having demonstrated these electrolyzers under demanding Martian conditions, we intend to also deploy them under much milder conditions on Earth to utilize brackish or salt water feeds to produce hydrogen and oxygen, for example, through seawater electrolysis.”