Electricity
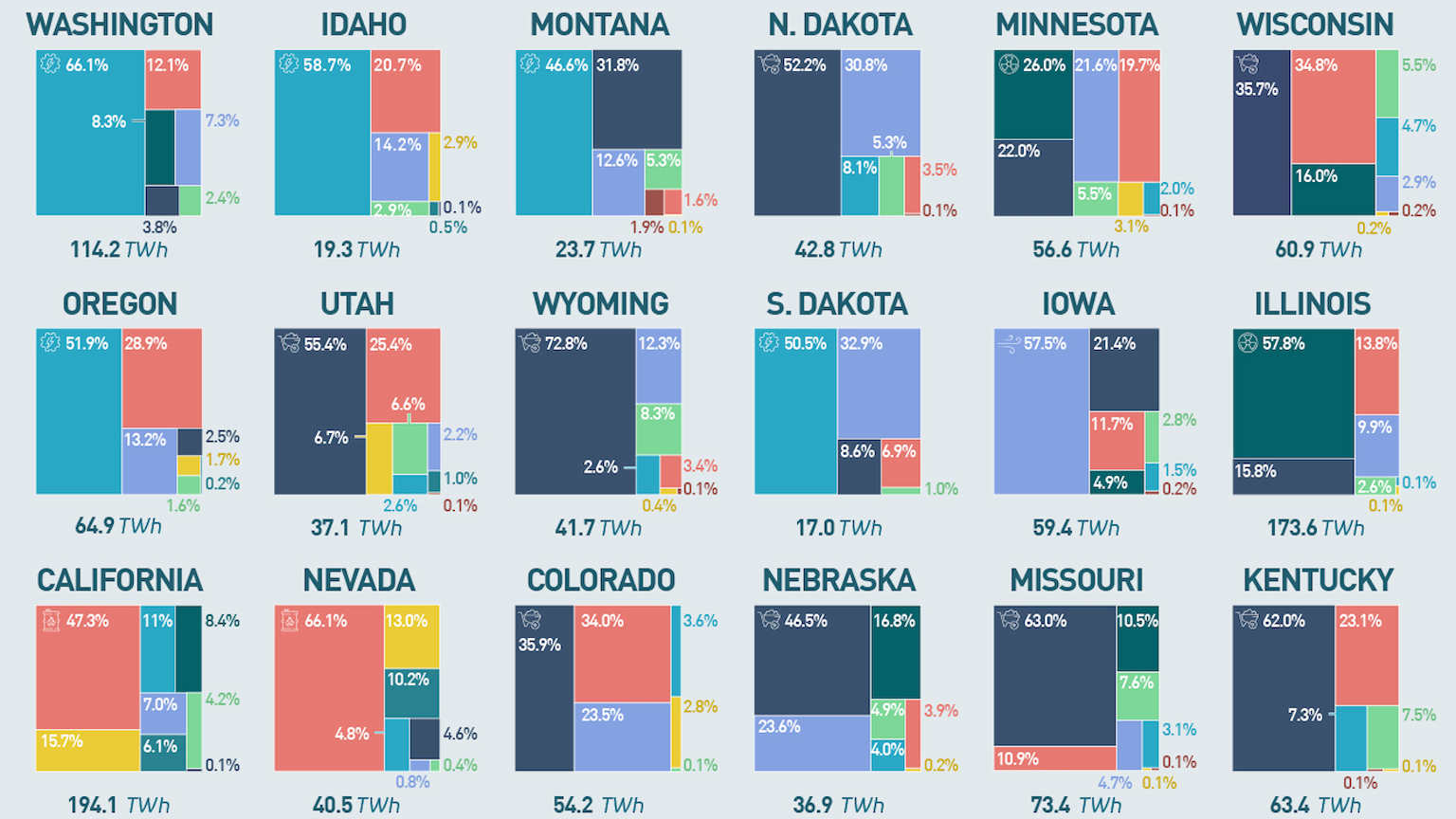
U.S. states vary radically in terms of electricity generation. Vermont is the cleanest, while Delaware is the dirtiest.
Scientists are solving the problem of costly energy storage.
This everyday electrical phenomenon had no widely accepted scientific explanation — perhaps, until now.
While we can see many solar storms coming, some are “stealthy.” A new study shows how to detect them.
How one startup plans to use “death rays” for good instead of evil.
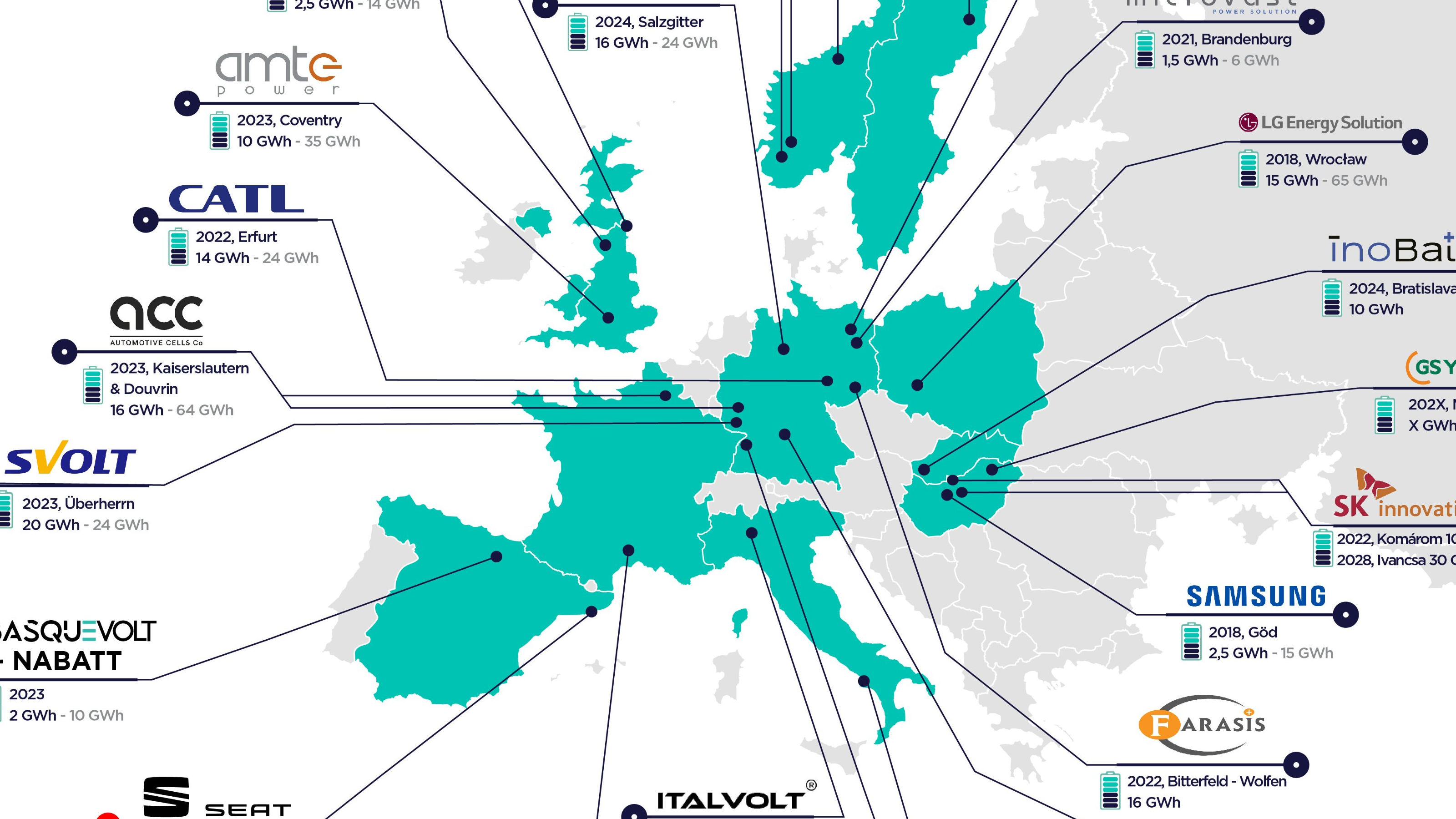
Map shows Europe’s imminent Great Leap Forward in battery cell production
It’s like a little magnetic “nom, nom.”
While it’s always been a boon to Popeye’s “muskles,” it looks like spinach may also have a role to play in clean future batteries.
Dust sticking to things on the moon is a serious problem researchers are trying to solve.
Utilizing nuclear waste converted to diamonds, this company’s batteries will reportedly last thousands of years in some cases.
Your television may soon get a serious upgrade.
By leveraging the difference between lit and shadowed areas, a new energy source perfect for wearables is invented.
Device for harnessing terahertz radiation might enable self-powering implants, cellphones, other portable electronics.
A microbial organism pulls electricity from water in the air.
Ready to become a tech wizard? Creation Crate’s electronic projects are delivered to your door with everything you need to start building and learning.
A new device shows promising results in its ability to convert CO2 and water into useful fuels.
You’ve likely heard of solar energy, but what is osmotic energy?
The racing plane is hoped to be the fastest electric plane in existence.
If you’ve ever had an affinity for machines or just wanted to know how to fix faulty household gadgets on your own, then learning the basics of electrical engineering might be up your alley.
Can changing who delivers your electricity to you solve a slew of problems?
A mysterious startup reveals a groundbreaking solar energy achievement.
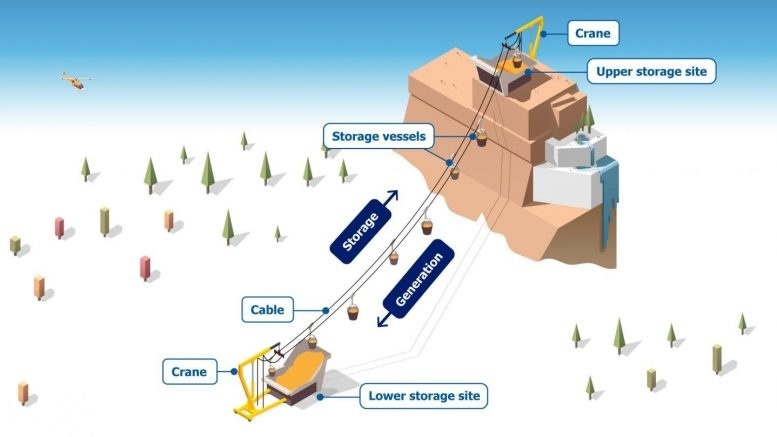
Researchers propose a gravity-based system for long-term energy storage.
Researchers evaluated the best and worst ways to remove greenhouse gases from the atmosphere in a recent report.
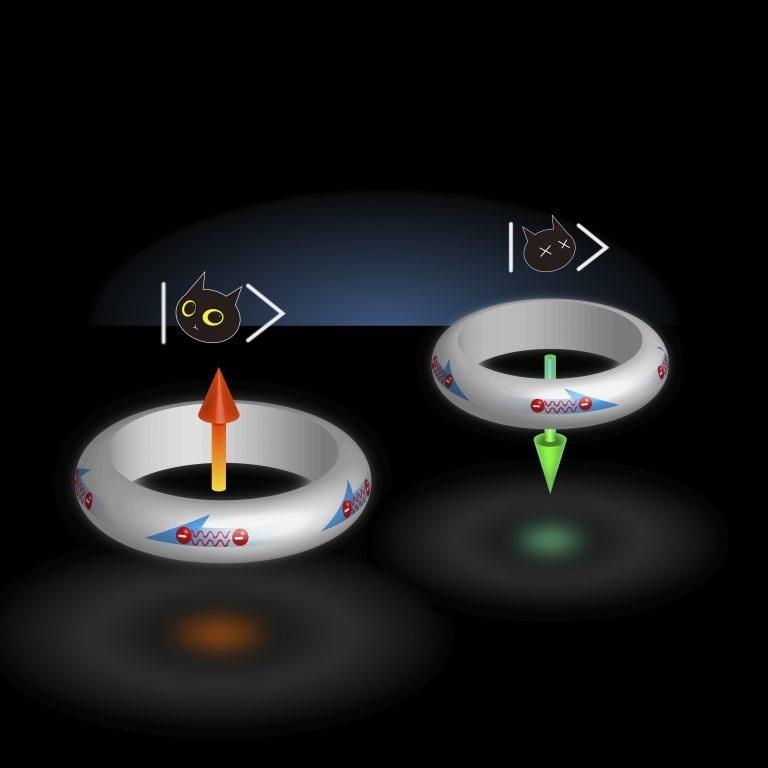
Scientists from John Hopkins find a material for quantum computing.
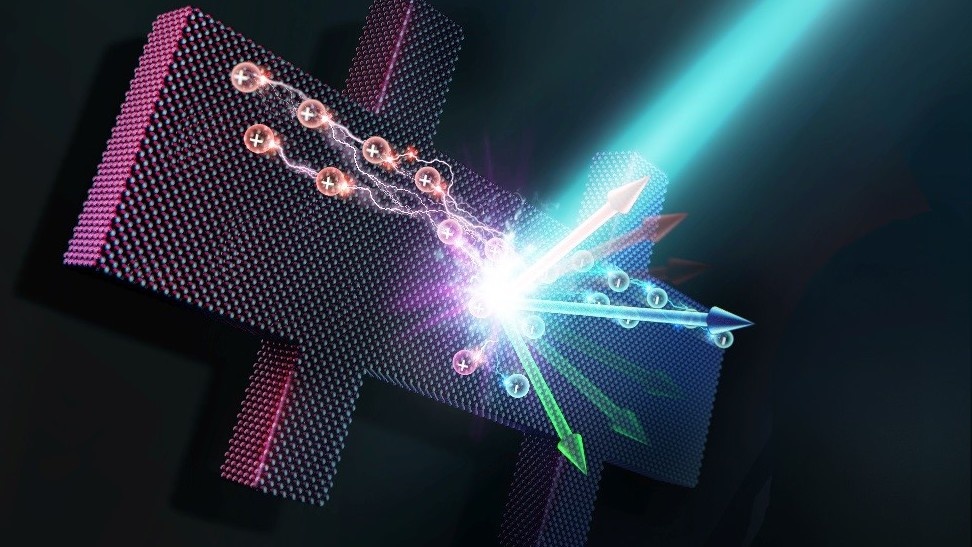
Scientists discover the inner workings of an effect that will lead to a new generation of devices.
The national public charging infrastructure is coming online.
Such a battery would make it far cheaper to implement robotaxis and long-haul electric trucks, both of which Tesla is developing.
Researchers from UCLA invent a device that generates electricity from a rather unusual source.
Can you make solar power work when the sun goes down? You can, and Dubai is about to run a city that way.
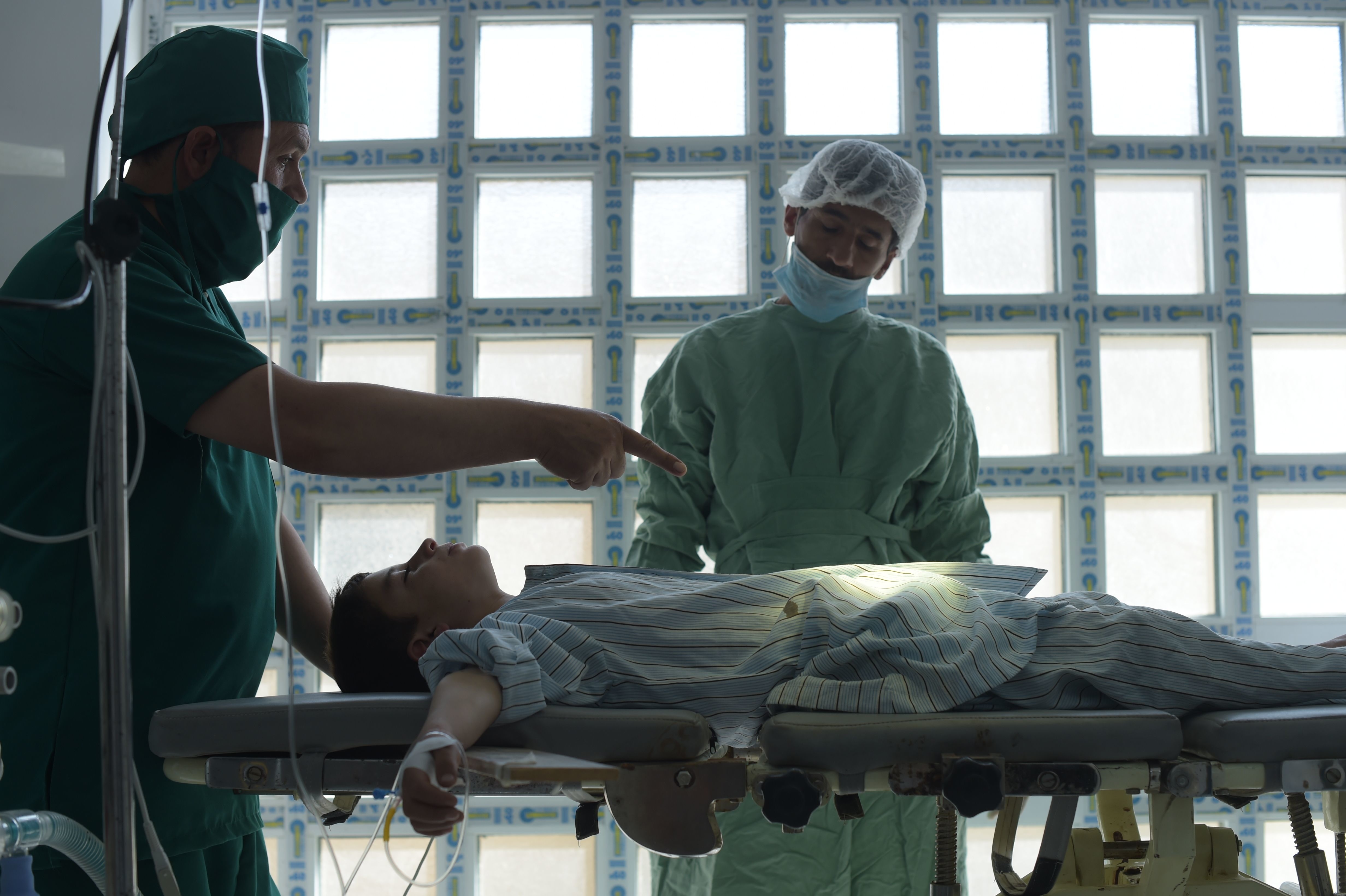
Doctors may be able to painlessly reshape cartilage with the technique.