bacteria
The discovery could help astronauts find better ways to grow food in space.
Three lines of evidence point to the idea of complex, multicellular alien life being a wild goose chase. But are we clever enough to know?
Starling flocks, schools of fish, and clouds of insects all agree.
For the first time, it was discovered that nonphotosynthetic bacteria have a circadian clock.
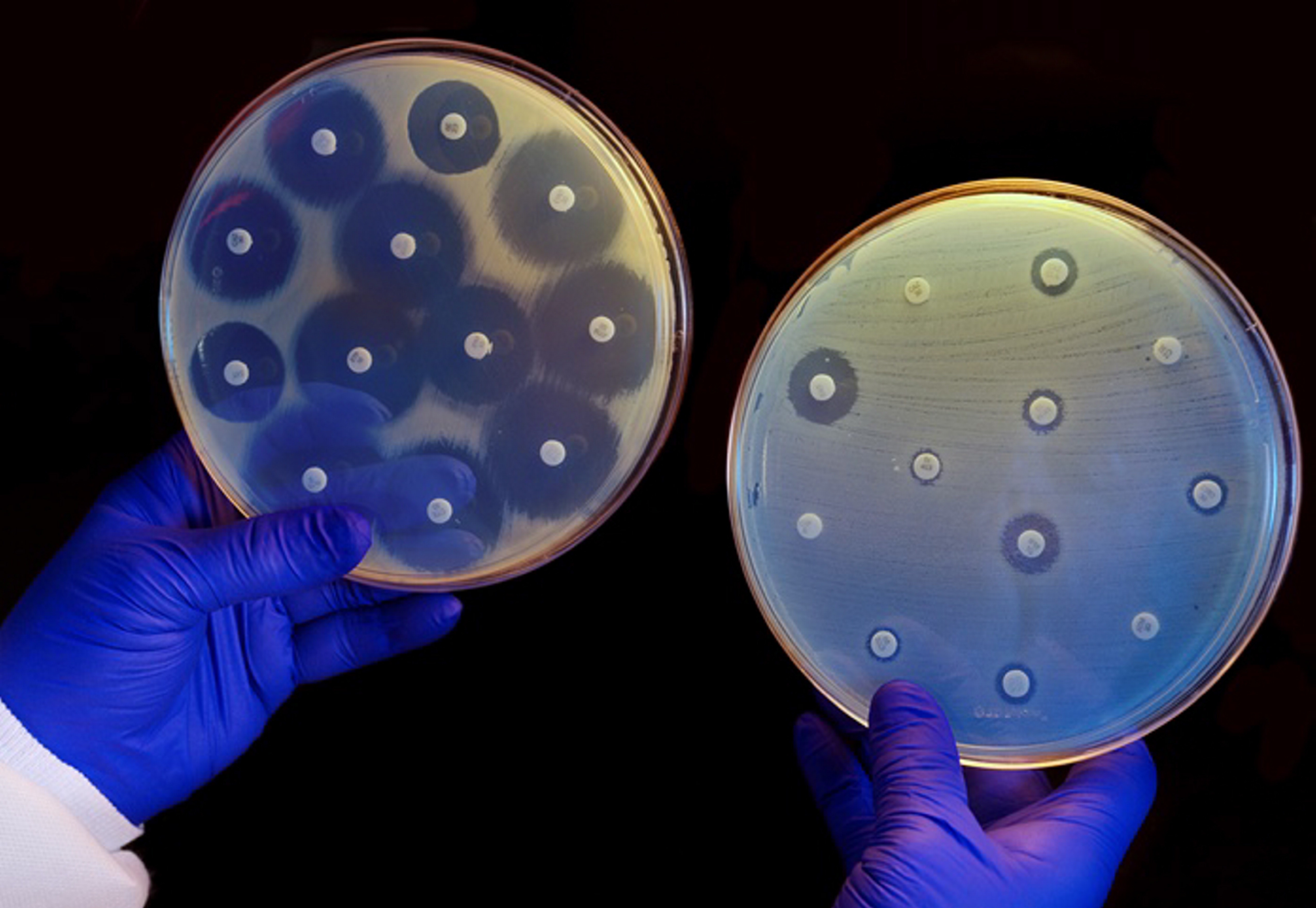
A new antibiotic hits germs with a two pronged attack.
The microbes that eventually produced the planet’s oxygen had to breathe something, after all.
An intriguing theory explains animals’ magnetic sense.
Various studies examine the impact of humidity, temperature, rain, and sunshine on COVID-19.
A new study shows bacteria could survive travel from Earth to Mars.
German researchers have just solved the mystery of how these substances work.
Smart bandages quickly identify antibiotic-resistant bacteria, and normal bacteria, in owies.
The physical action of handwashing plus the properties of soap is a one-two punch for the virus.
▸
1 min
—
with
Most homes are using insufficient methods to determine when chicken is done cooking and safe to eat.
Dr. Kate Biberdorf explains why boiling water makes it safer and how water molecules are unusual and cool.
▸
3 min
—
with
A drug developed to combat Alzheimer’s Disease can trigger regeneration of tooth dentin.
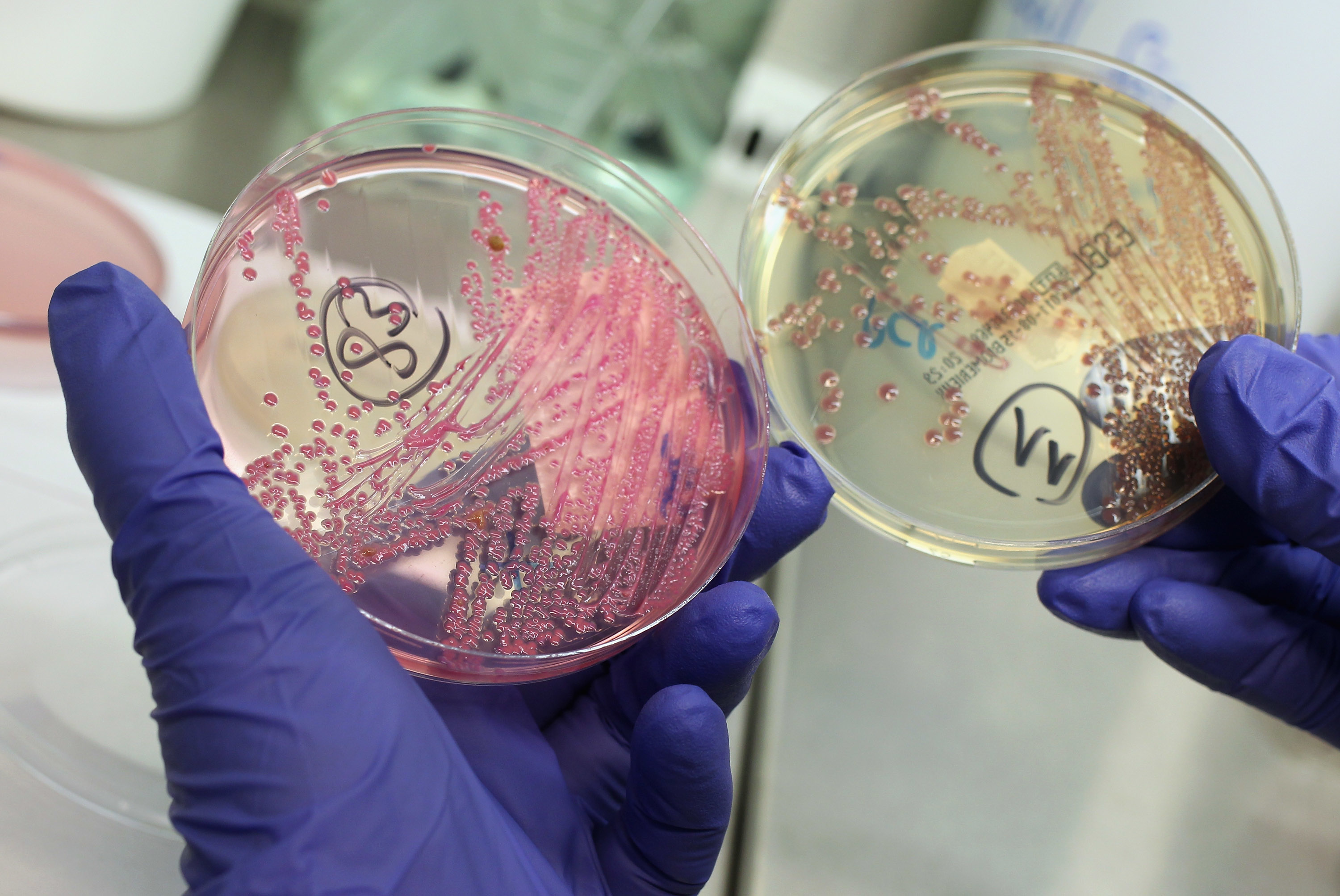
A deep-learning model identifies a powerful new drug that can kill some antibiotic-resistant bacteria.
A new hypothesis suggests that you can “catch” noncommunicable diseases from other people via the microbiome.
Antibiotic resistance poses one of the biggest threats to global public health.
The premier hospitals tend to have the most superbugs — they also have the best experts.
▸
2 min
—
with
What you eat — and when — can make you superhuman.
▸
12 min
—
with
The reason one diet does not suit all may be found in our guts.
Bats are being subjected to a deadly plague that may be threatening their existence. However, a new bacterial spray may help fight the fungus responsible.
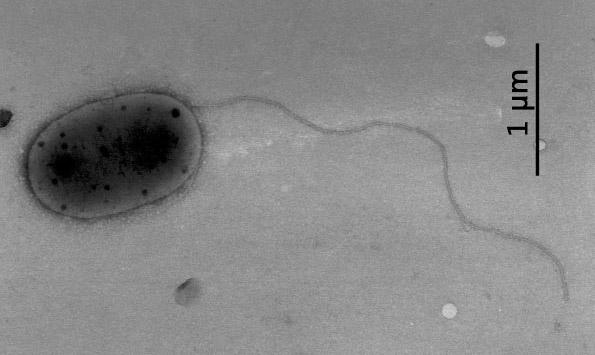
Meet Deinococcus radioduranst, the world’s toughest bacteria.
Here’s how we stop a health crisis before it wreaks havoc on us.
▸
15 min
—
with
Stems cells have always been pretty amazing.
Can dirt help us fight off stress? Groundbreaking new research shows how.
Cornell University researchers propose using biology to transform the storage of sustainable energy.
A new study has identified 12 times as many viral populations as previous research.
Antibiotics are often the fastest and simplest way for doctors to help their patients. But with the threat of drug-resistant bacteria, how do we stop prescribing antibiotics?
They may be using an “air bridge” to do so.