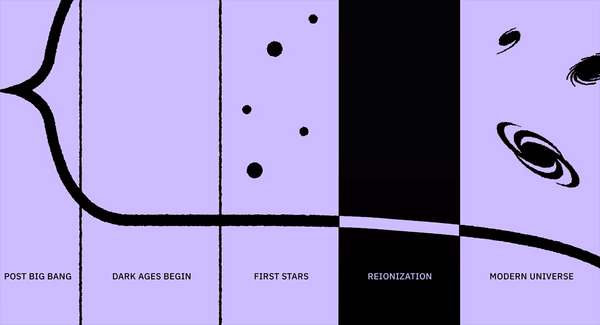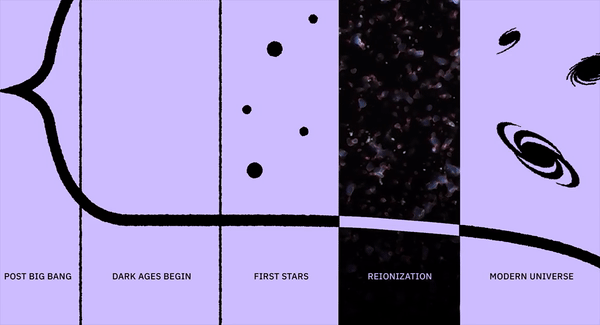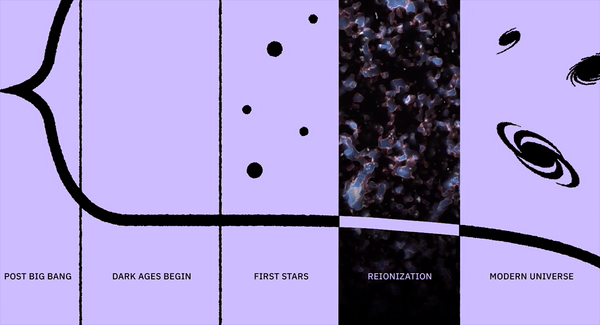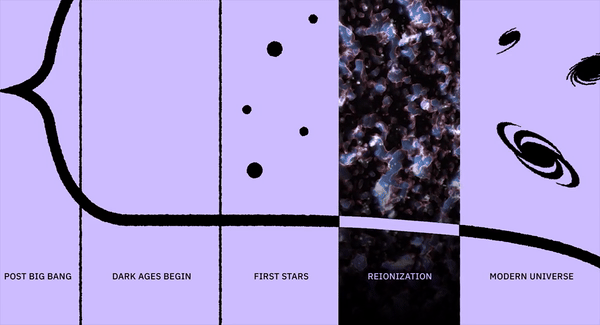
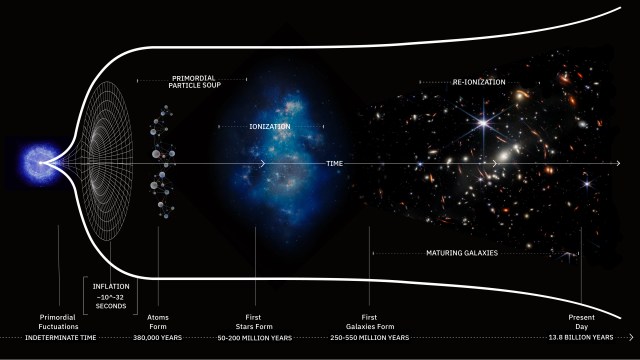
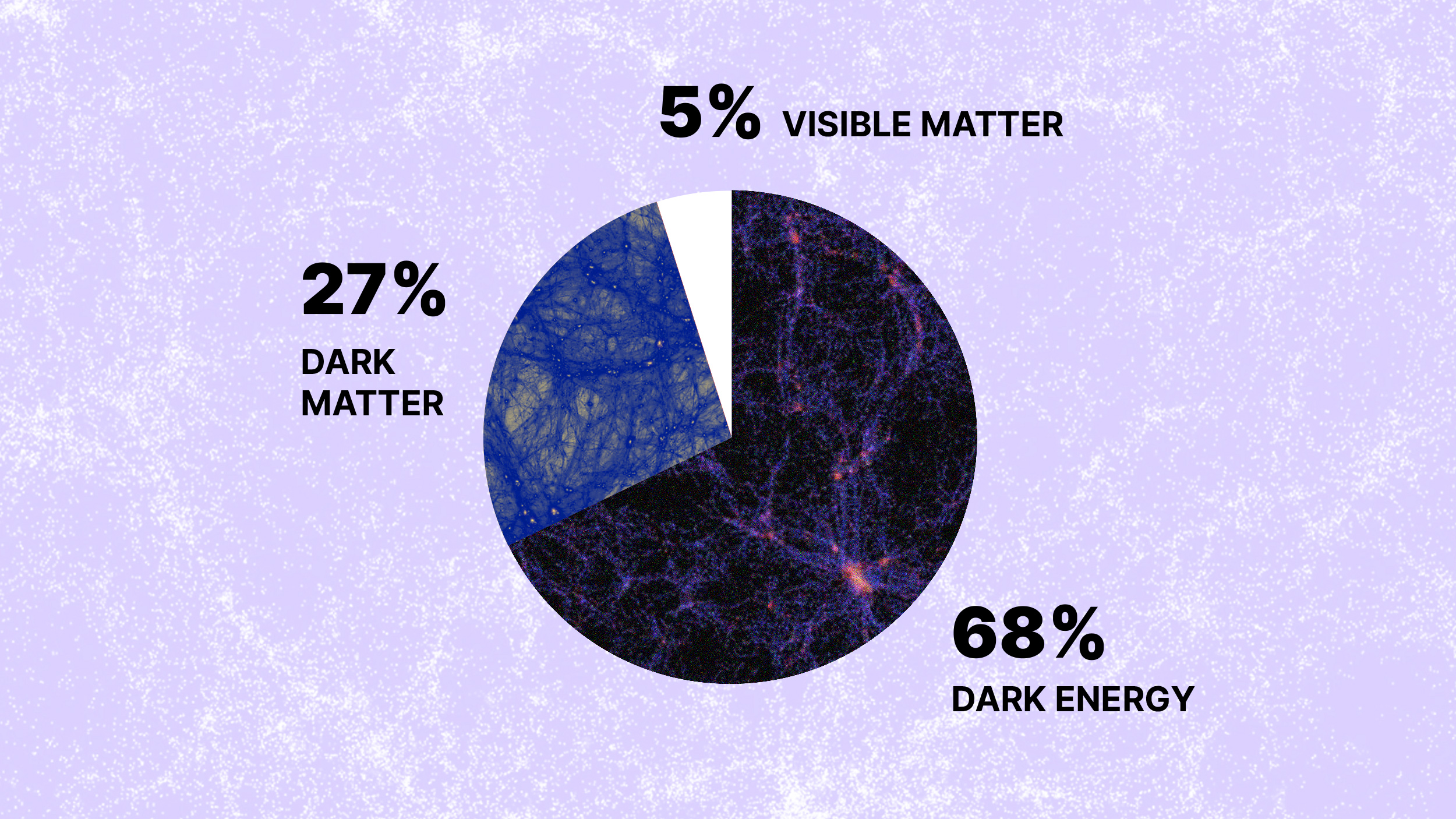
What was it like when life first sprang forth on Earth?

- Early Earth was incredibly toxic, full of volatile chemicals, volcanic pollutants, and high temperatures that would have sterilized any organic processes that spontaneously occurred.
- But after hundreds of millions of years, our planet cooled, water collected in liquid form on the surface, and the environment became somewhat stable.
- In an unremarkable pool of shallow water, a complex chemical reaction created something that could both metabolize abundant nutrients and replicate itself, and with it, the beginnings of life emerged on Earth.
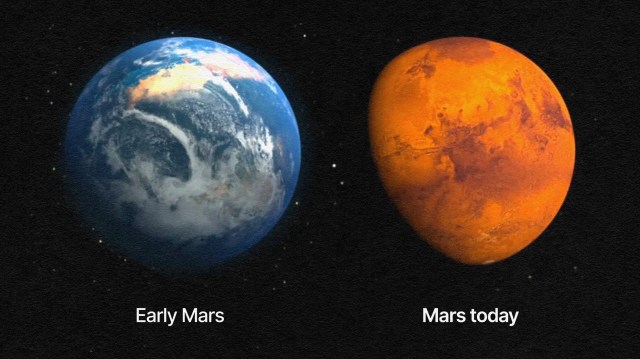
If you came to our Solar System right after it formed, you would have seen a completely foreign-looking sight. Our Sun would have been about the same mass it is today, but only a fraction as luminous, as stars heat up and shine more brightly as they age. The four inner, rocky worlds would still be there, but three of them would look extremely similar. Venus, Earth, and Mars all had thin atmospheres, the capacity for liquid water on their surfaces, and the organic ingredients that could give rise to life. Earth had a large, close moon, Mars had three, while Venus had none, as far as we can tell. Even though all of these worlds were hot and volcanically active, they all had almost completely given up their primordial, hydrogen-and-helium-rich envelopes, as photoevaporation had boiled them all away.
While we still don’t know whether life ever took hold on Venus or Mars, we know that by the time Earth was only a few hundred million years old — possibly after as little as 100 million years but no later than 700 million years — there were organisms living on its surface. After billions of years of cosmic evolution gave rise to the elements, molecules, and conditions from which life could exist, our planet became the one where life not only arose, but where it continued to thrive for billions of years subsequently, even giving rise to us, humanity, as part of its cosmic story. To the best of our scientific knowledge, here’s what those first steps of life’s emergence were like.
Life — at least, life as we know it — has a few properties that pretty much everyone agrees on. While life on Earth involves carbon-based chemistry (requiring carbon, oxygen, nitrogen, hydrogen, and many other elements like phosphorus, copper, iron, sulfur, and so on) and relies on liquid water, other combinations of elements and molecules may be possible. The four general properties that all living organisms share, however, are as follows:
- Life has a metabolism, where it harvests energy/resources/nutrients from an external source for its own use.
- Life responds to external stimuli from its environment, and alters its behavior accordingly.
- Life can grow, adapt to its environment, or can otherwise evolve from its present form into a different form.
- And life can reproduce, creating viable offspring that arise from its own internal processes.
Although different camps of biologists frequently argue over the particulars of these points in considering whether something is or isn’t alive (viruses are probably the most hotly debated in-between case), the consensus at present is that all four of these must be in place, simultaneously, for a population of organisms to be considered alive.
Snowflakes and crystals may be able to grow and reproduce, but their lack of a metabolism prevents them from being classified as alive. Proteins may have a metabolism and be able to reproduce, but they do not respond to external stimuli or alter behavior based on what they encounter. Even viruses can only reproduce by infecting other successfully living cells and using them as a host, casting doubt on whether they’re classified as living or non-living.

At the same time, no one doubts that the raw ingredients required for life to form exist practically everywhere we’ve been able to look. Many organic materials — chemical compounds like sugars, amino acids, ethyl formate, and even complex ones like polycyclic aromatic hydrocarbons — are found in interstellar space, in asteroids, and were abundant on early Earth. All five of the nucleobases used in biological processes on Earth have been found aboard asteroids, and in addition to the 22 amino acids that are leveraged in biochemical activity on our world, perhaps 60 other species of amino acid, including amino acids of the opposite chirality to the ones playing a role in life processes on Earth, have been found within meteorites that we’ve subsequently examined in the lab.
However, despite everything we’ve learned about prebiotic ingredients found in interstellar space, around other stars, and in the primitive relic materials remaining here in our own Solar System, there is no evidence for any biological activity outside of Earth. Although many have been tempted to suggest that life may have begun even in the environment of interstellar space, we have no evidence of any type that indicates life began prior to Earth’s formation.
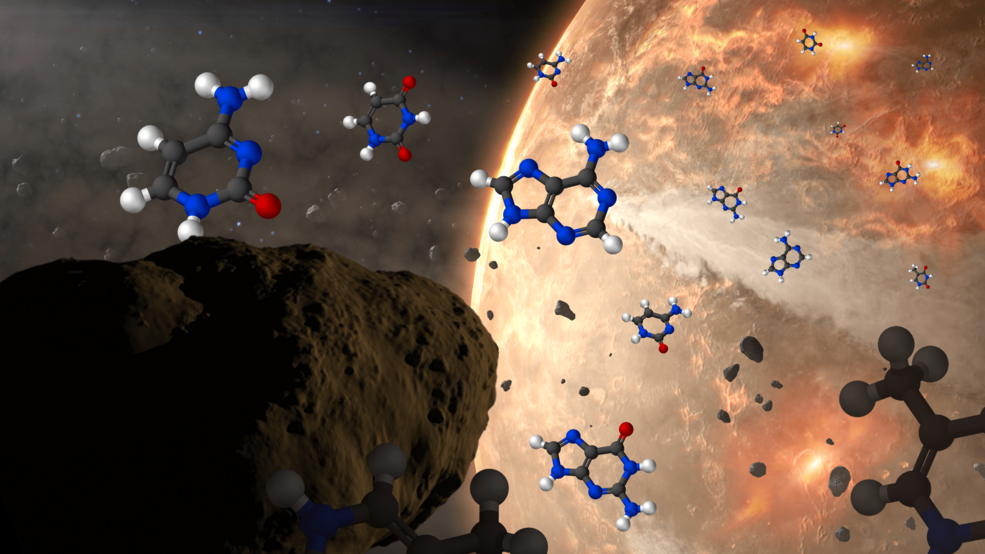
Instead, the leading thought is that the Earth was formed with all of these raw ingredients on it, in addition to perhaps many more that could have later been included in life processes. Perhaps full fledged nucleotides were common; perhaps various proteins and protein fragments came early and already pre-assembled; perhaps lipid layers and bilayers would spontaneously arise in an aqueous environment of liquid water. All of those remain possibilities that should be considered, but that have yet to be proven or demonstrated. Life’s precursors, in whatever state of simplicity or complexity they happened to exist in, were certainly present and abundant in the environment of early Earth.
In order to go from precursors to life to actual life, however, it’s believed that we needed the right environment to facilitate life’s emergence from non-life. All three of the planets with initially favorable conditions for life to arise — Venus, Earth, and Mars — all had:
- a reasonable level of surface gravity,
- initially thin atmospheres,
- an environment that admitted liquid water on their surfaces,
- and all of these biochemical precursor molecules.
While all three worlds likely had a chance to form life for the first time, Earth is the only such world where we have abundant evidence that life not only did arise, but arose relatively early on in our Solar System’s history.
Shortly after planetary formation, these molten-surface worlds were too hot for liquid water to stably exist on their surfaces, as the interiors of these planets require long, geological periods of time to cool. Early on, volcanic activity was rampant, and the surface was wildly unstable. At the same time, there was a period of heavy bombardment in our Solar System that impacted all bodies at these early times: where asteroid-like and comet-like objects struck each and every one of them, scarring their surfaces and creating pathways for sub-surface magma to emerge from below.
However, these two activities — volcanism and impacts — also bring water with them to the surface of these planets, with scientists still debating which mechanism is primarily responsible for delivering the majority of water to early Earth (and also Mars and Venus). The amount of water present, even early on, was very likely enough to create oceans, seas, lakes, and rivers, but not enough to create a waterworld, where they were completely covered in liquid water. Each of these planets likely possessed continents and oceans, and at the interface of the two, pools of water formed: regions where water can stably exist on dry land and be subject to all sorts of energy gradients. In addition, freshwater melt, including in regions where there were copious levels of volcanic activity beneath, created stable pools of water with very low levels of salt.

Think about these conditions together. In pools of water where abundant minerals line the surface, where heat and energy gradients are abundant (from geological heat as well as from direct sunlight and the night/day cycle), molecules and atoms can bind together. Amino acids can link up to form proteins, dissolved ions can turn those proteins into enzymes, and cycles of evaporation can dehydrate numerous types of molecules, compelling them to link together. Additional geochemical phenomena — including precipitation, porous fluid flow in the presence of minerals, and gradients of water activity — could all provide opportunities for molecules to bind together in novel and interesting ways.
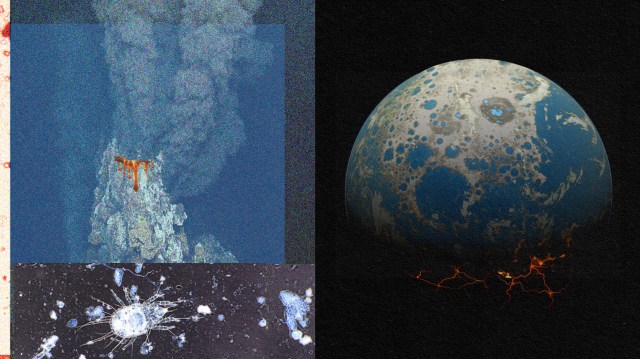
The effects of tides on Earth may be enhanced by the Moon, but even without moons, Venus, Earth, and Mars all possess tides due to the Sun. (About a quarter to a third of Earth’s tidal activity is due to the Sun.) However, there’s an additional energy source that the Earth possesses that likely contributed to life’s origin, that may not have been as spectacular on Venus or Mars: thermal activity from the interior of the planet. At the bottom of the oceans, hydrothermal vents are geological hotspots that also provide excellent candidate locations for life to arise. Even today, they are home to organisms known as extremophiles: bacteria and other lifeforms that can withstand the temperatures that typically break the molecular bonds associated with life processes.

These deep sea vents contain enormous energy gradients as well as chemical gradients, where extremely alkaline vent water mixes with the acidic, carbonic-acid-rich ocean water. Finally, these vents contain both sodium and potassium ions, as well as calcium carbonate structures that could serve as potential templates for the first cells or proto-cells. The fact that life exists in environments like this points to worlds like Europa or Enceladus as possible homes for life elsewhere in even the modern Solar System.
But perhaps the most likely location for life to begin on Earth is the best of all worlds: hydrothermal fields. Volcanic activity doesn’t solely occur beneath the oceans, but also on land. Beneath areas of fresh water, these volcanically-active areas provide an additional heat and energy source that can stabilize temperatures and provide an energy gradient. All the while, these locations still allow evaporation/concentration cycles, provide a confined environment that enables the right ingredients to accumulate, while also allowing for a sunlight/night cycle of exposure.
On Earth, we can be confident that tidepools, hydrothermal vents, and hydrothermal fields were all common. While many of the precursor molecules certainly originated beyond Earth and were later brought here, it was likely right here, on planet Earth, that the transformation of non-life into life spontaneously occurred, with some sort of nucleic acid-peptide coevolution providing the most favored pathway to date.
It’s unlikely that the very first molecules that arose with the properties to metabolize energy from a source of nutrients and reproduce themselves, however, would survive and thrive for long. Without a membrane to gather nutrients and protect their inner workings from the harsh outside environment, many of these “metabolic replicators” likely went extinct in short order. It likely required that another step — perhaps even another leap — be taken before the ability to respond to external stimuli from its environment was acquired. Once that ability, plus the ability to grow, change, and/or evolve (which may already be in place as soon as nucleic acids exist) joins these metabolic replicators, we can pinpoint that moment and say that full-fledged life has truly arisen.
Over time, the Earth has changed tremendously, as have the living organisms on our planet. We do not know if life arose once, more than once, or in disparate locations. What we do know, however, is that if we reconstruct the evolutionary tree of every extant organism found on Earth today, they all share the same common ancestor. By studying the genomes of the extant organisms, biologists can reconstruct the timescale of what’s known as LUCA: the Last Universal Common Ancestor of life on Earth. By the time the Earth was less than 1 billion years old, life already had the ability to transcribe and translate information between DNA, RNA, and proteins, and these mechanisms exist in all descended organisms today. Whether life arose multiple times is unknown, but it is generally accepted that all life forms present today have indeed descended from a single population.

Despite the fact that geological processes can often obscure the fossil record beyond a few hundred million years, we have been able to trace back the origin of life extraordinarily far. Microbial fossils have been found in sandstone dating to 3.5 billion years ago. Graphite, found deposited in metamorphosed sedimentary rock, has been traced back to having biogenic origins, and dates back to 3.8 billion years ago. There are very, very few pieces of the geological record that date back before this time, but we can be fairly certain — based on the most direct evidence available — that life was already thriving on planet Earth some 3.8 billion years ago. That’s impressive for a planet that only formed 4.5 billion years ago!
At even earlier, more extreme times, the deposits of certain crystals in rocks may have originated (this is more hotly debated) from biological processes, suggesting that Earth was teeming with life as early as 4.3 to 4.4 billion years ago: as soon as 100-200 million years after the Earth and Moon formed. If these zircon crystals, which have inclusions within them that may indicate the metamorphosed remains of organic material, really do come from life processes, the implications are astounding. It would mean that, even through the heavy bombardment period, life on Earth existed: perhaps almost for as long as planet Earth itself.
At some point on our planet, in the very early stages, it became rich in precursor molecules that have the potential, if combined in just the right way, to give rise to life. Under the right environmental and chemical conditions, those molecules joined together in a fashion that allowed them to perform two important tasks simultaneously:
- to metabolize energy,
- and to reproduce or replicate themselves.
At some point down the line, those early metabolic replicators, perhaps already classifiable as life or perhaps requiring more to truly be considered “alive,” gained the ability to respond to their environments, as well as to grow, adapt, and even to evolve. Even if those primitive forms of life would be unrecognizable to us today, and even though we don’t know for certain the exact mechanism by which it occurred, those events truly represent the origin of life on Earth. In a radically unbroken string of biological successes, our planet has been a living world ever since.
While Venus and Mars may have had similar chances, radical changes to Venus’ atmosphere rendered it a searing hothouse world after as little as merely 200-300 million years, while the death of the Martian magnetic field caused its atmosphere to be stripped away, rendering it solid and frozen after approximately 1-1.5 billion years. While asteroid strikes may have subsequently sent Earth-based life off-world, where it may yet travel all throughout the Solar System and even the Milky Way galaxy, all the evidence we have suggests that planet Earth, right here, is where the biological activity that Earth is home to got its start.
It took somewhere between 9.4-to-10.0 billion years after the Big Bang for planet Earth to go from a barren, lifeless state to one that was teeming with life. In all the time since, we’ve never looked back.