FDA approves first drug that prevents migraines. Is the price tag worth it?
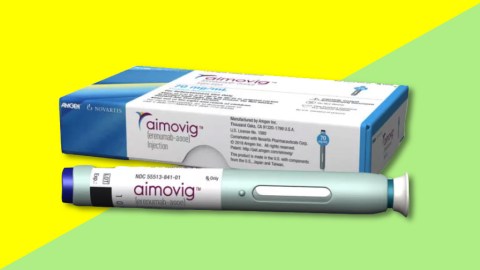
The Food and Drug Administration has approved a drug designed to prevent migraines, marking a first for the pharmaceutical industry and the millions of Americans who suffer from the chronic, debilitating disorder.
The new drug, Aimovig, is a once-monthly self-injection, and biopharmaceutical company Amgen will sell it for $6,900 a year.
“Aimovig provides patients with a novel option for reducing the number of days with migraine,” said Eric Bastings, M.D., deputy director of the Division of Neurology Products in the FDA’s Center for Drug Evaluation and Research. “We need new treatments for this painful and often debilitating condition.”
Over the course of several studies, migraine sufferers who took Aimovig experienced an average of 1 to 2.5 fewer migraine days per month. It’s not quite a cure-all, but the drug will likely be welcomed by the 4 million Americans who have migraines at least 15 days a month.
Never experienced a migraine? Here’s a description of what one feels like:
“Put your finger on your temple and imagine drilling it inside your head,” said a 29-year-old woman named Heather to Prevention. “My migraines feel like a screwdriver in there, in that one spot, always on my left side and in my left eye. I get a burning sensation throughout my body and in my jaw. Everything becomes sensitive to the touch, like my muscles are on fire.”

Image: aka Tman via Flickr
Migraines are also often accompanied by nausea, vomiting, and hypersensitivity to light and sound, and they can be triggered by stress, hormonal changes, flashing lights, changes in sleep or diet. Women are three times as likely to experience chronic migraines, and the disorder affects about 10 percent of people worldwide.
In the 1980s, researchers observed that people who suffer from migraines produced too much of a protein fragment called CGRP. The new drug is an antibody that effectively blocks some of that CGRP but allows the rest to perform its normal functions in the body. Still, not much is known about what exactly CGRP does, as is evidenced by a rather vague description on the Amgen website:
“There may be a connection between CGRP, CGRP receptors, and migraine.”
In any case, the annual price tag of $6,900 might seem steep, but it comes as a surprise to some analysts who expected it to be $10,000 or more. It’s predicted that insurers will reimburse patients for much of the costs.
“The payers recognize that there is a clear and longstanding unmet need in migraine,” said Tony Hooper, executive vice president of global commercial operations at Amgen, who added that pharmacy-benefit managers and insurers are mostly “supportive of our price.”