stem cells
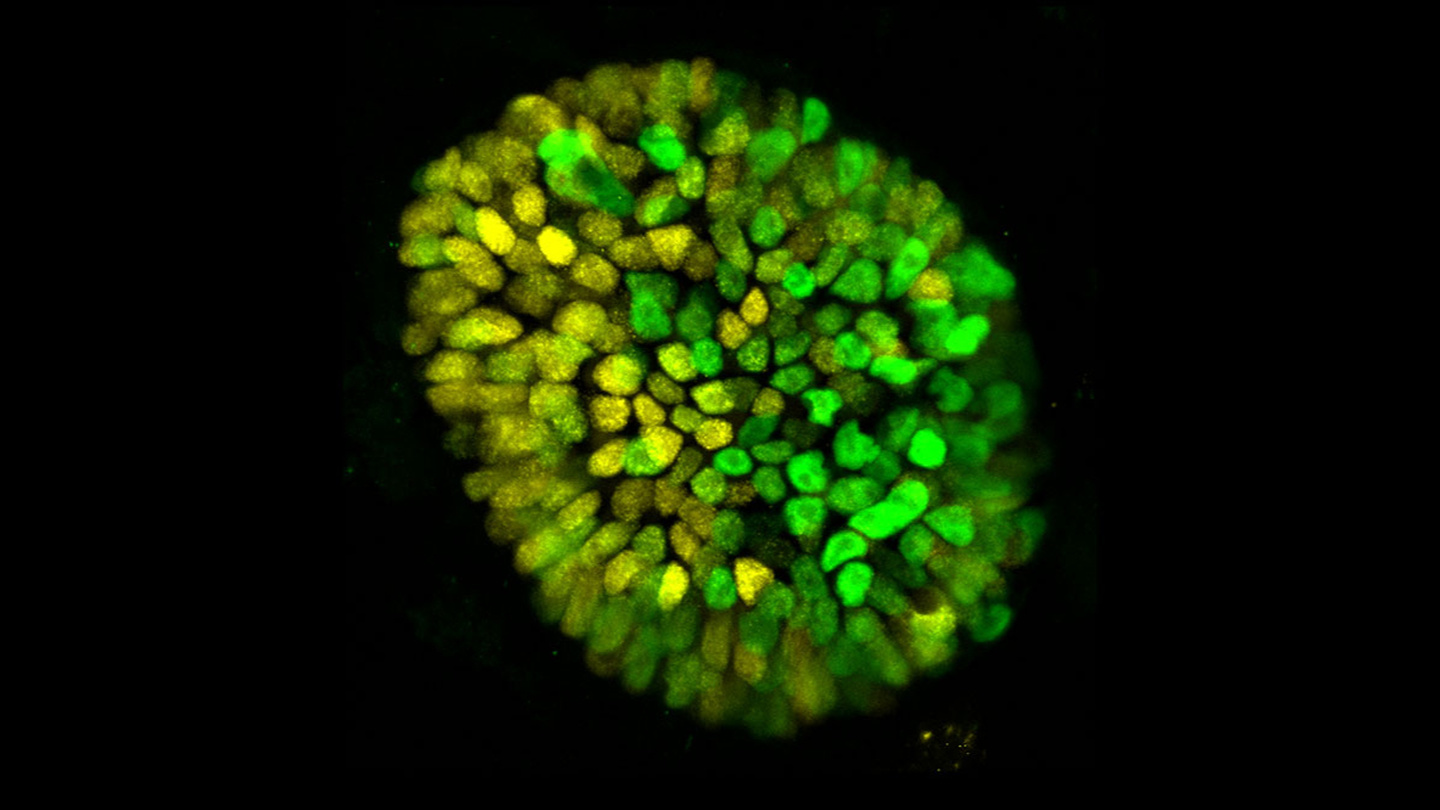
A unique 3D model allows researchers to explore embryonic development.
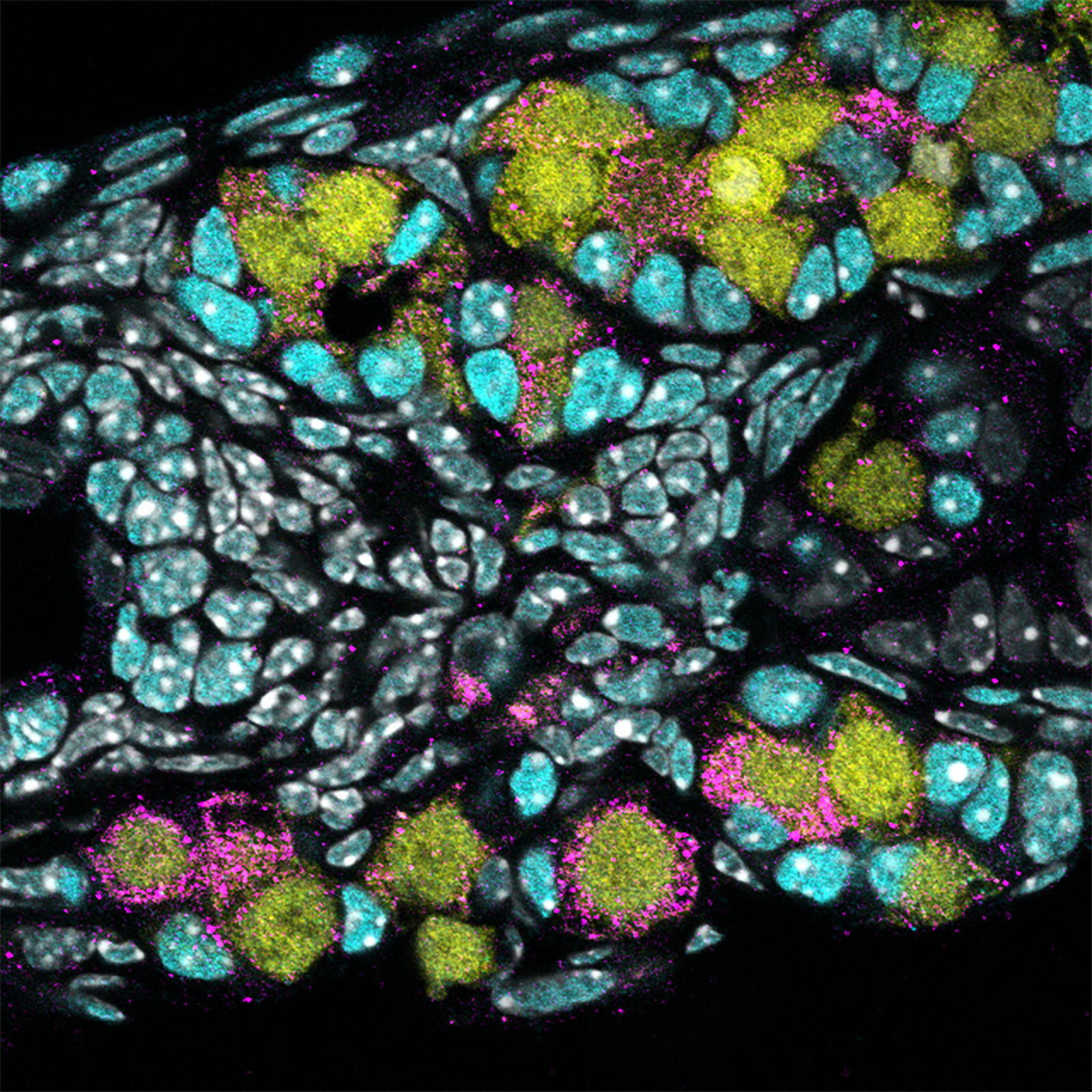
Stems cells have always been pretty amazing.
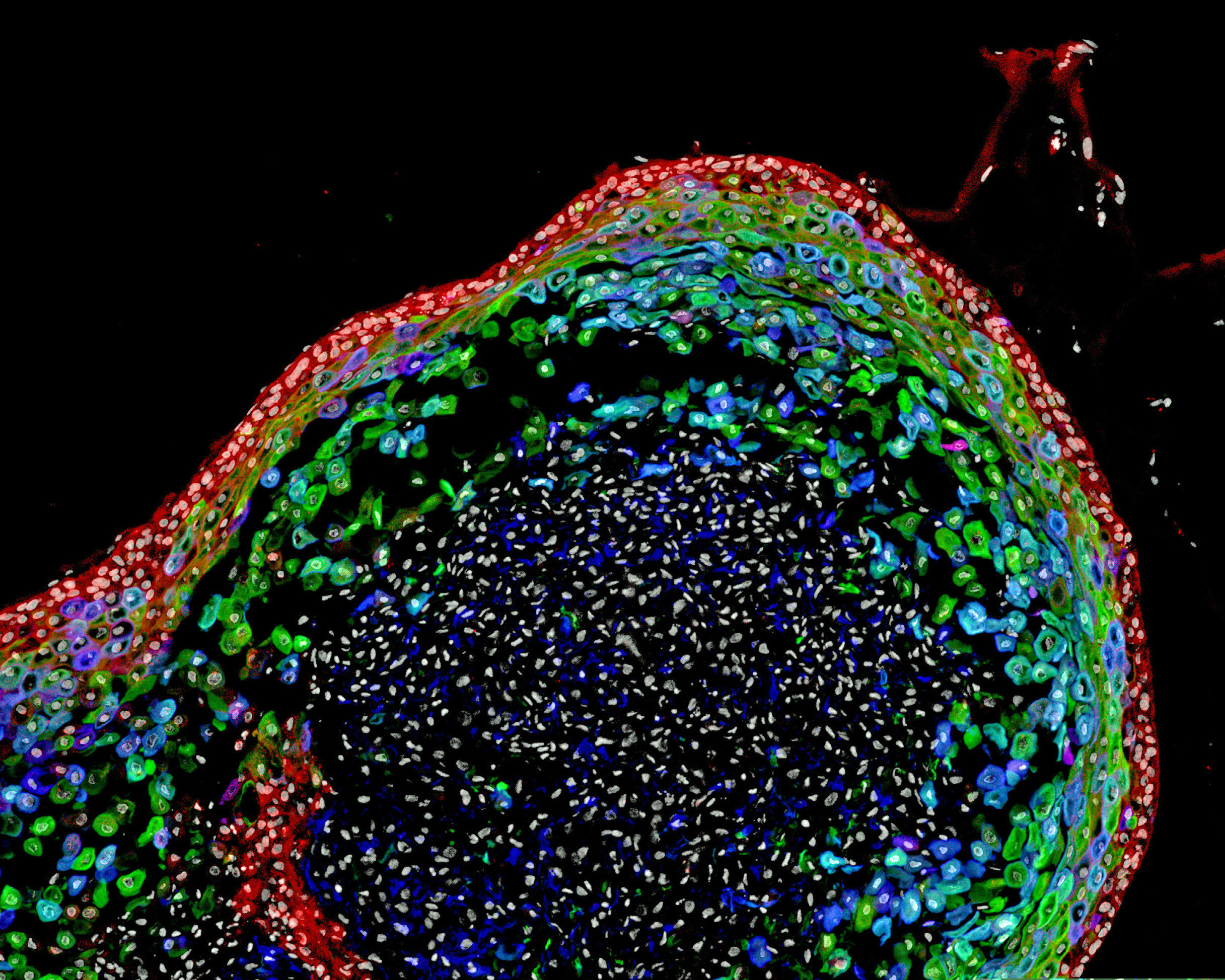
Scientists have grown a model human esophagus using pluripotent stem cells for the first time.
Human egg cells can now be created from donor blood — a brave new world is upon us.
It’s a development that could one day lead to much better treatments for osteoporosis, joint damage, and bone fractures.
Stem cells have endless uses. This study suggests they can even bring half dead teeth back to life.
The results of two human clinical trials involving elderly patients suffering from frailty showed no adverse side effects and “remarkable” physical improvement.
Has technology advanced enough that we could stitch together body parts and reanimate the dead? Bill Nye one-ups that old-school Frankenstein vision with newer (and cooler) scientific possibilities.
▸
5 min
—
with