The Sun’s Energy Doesn’t Come From Fusing Hydrogen Into Helium (Mostly)

It does undergo nuclear fusion, but there are more reactions and more energy released from reactions other than H → He.
“The sun is a miasma
Of incandescent plasma
The sun’s not simply made out of gas
No, no, no
The sun is a quagmire
It’s not made of fire
Forget what you’ve been told in the past” –They Might Be Giants
If you start with a mass of hydrogen gas and bring it together under its own gravity, it will eventually contract once it radiates enough heat away. Bring a few million (or more) Earth masses’ worth of hydrogen together, and your molecular cloud will eventually contract so severely that you’ll begin to form stars inside. When you pass the critical threshold of about 8% our Sun’s mass, you’ll ignite nuclear fusion, and form the seeds of a new star. While it’s true that stars convert hydrogen into helium, that’s neither the greatest number of reactions nor the cause of the greatest energy release from stars. It really is nuclear fusion that powers the stars, but not the fusion of hydrogen into helium.

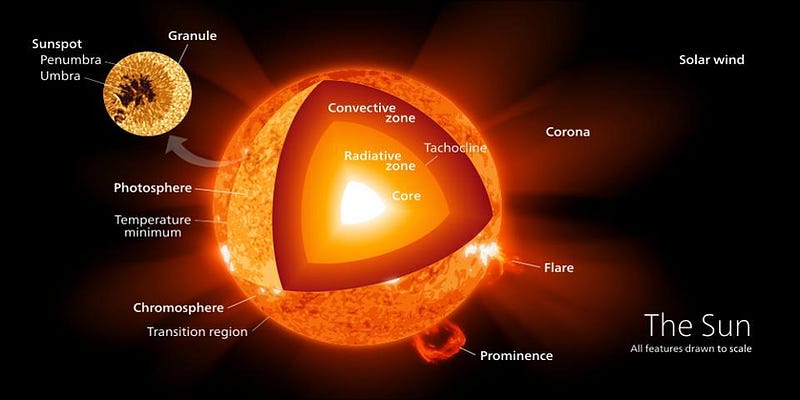
All stars, from red dwarfs through the Sun to the most massive supergiants, achieve nuclear fusion in their cores by rising to temperatures of 4,000,000 K or higher. Over large amounts of time, hydrogen fuel gets burned through a series of reactions, producing, in the end, large amounts of helium-4. This fusion reaction, where heavier elements are created out of lighter ones, releases energy owing to Einstein’s E = mc2. This occurs because the product of the reaction, helium-4, is lower in mass, by about 0.7%, than the reactants (four hydrogen nuclei) that went into creating it. Over time, this can be significant: over its 4.5 billion year lifetime thus far, the Sun has lost approximately the mass of Saturn through this process.

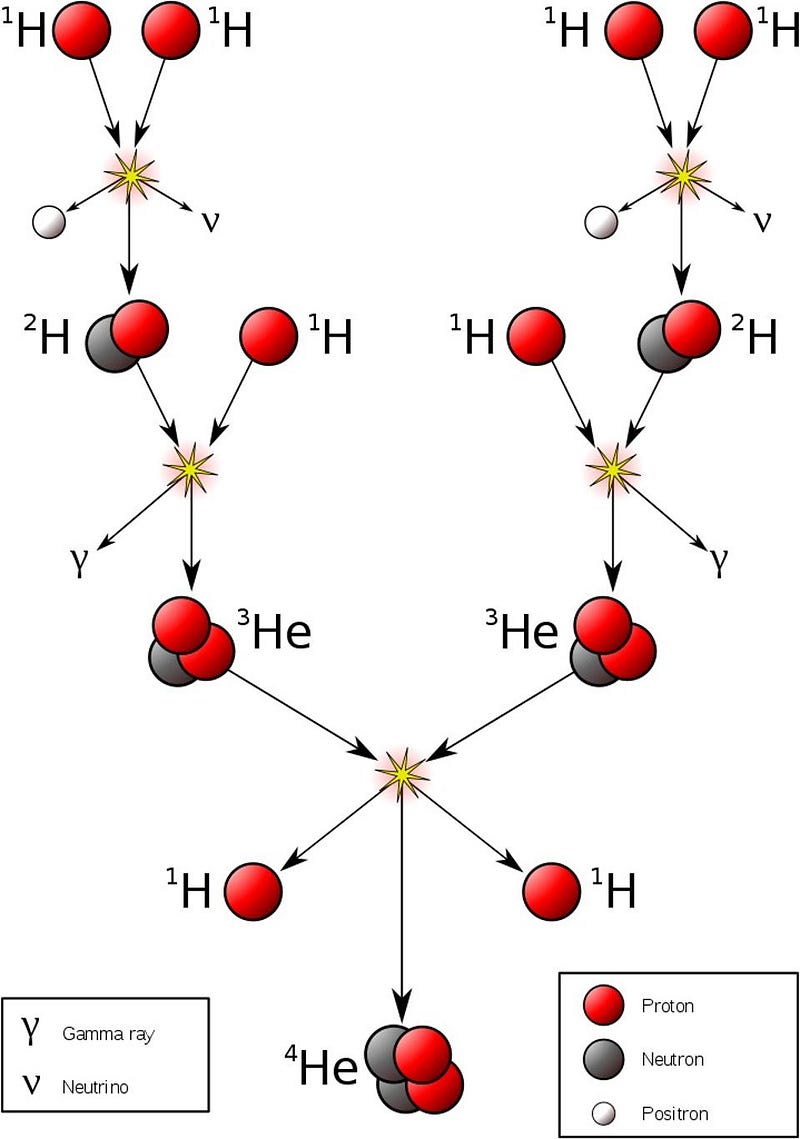
But the way it gets there is complicated. You can never have more than two objects collide-and-react at once; you can’t simply put four hydrogen nuclei together and turn them into a helium-4 nucleus. Instead, you need to go through a chain reaction to build up to helium-4. In our Sun, that involves a process called the proton-proton chain, where:
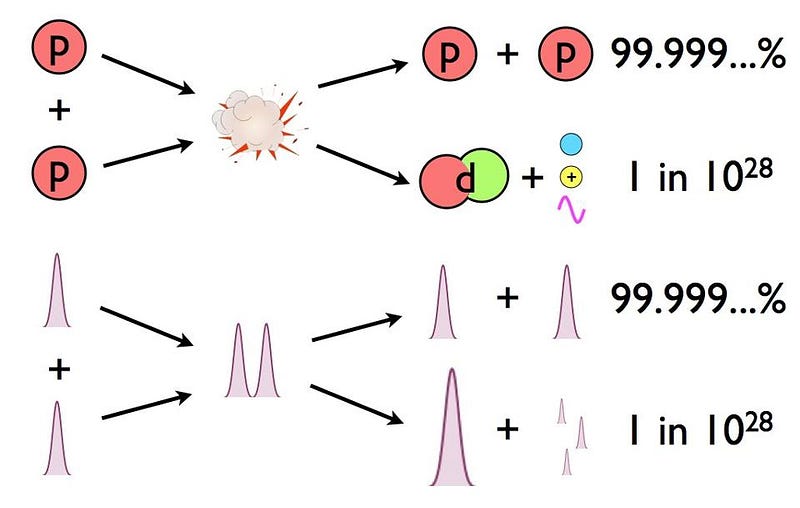
- Two protons fuse together to form a diproton: a highly-unstable configuration where two protons temporarily create helium-2,
- A tiny fraction of the time, one-in-10,000,000,000,000,000,000,000,000,000 times, that diproton will decay to deuterium, a heavy isotope of hydrogen,
- And it happens so quickly that humans, who can only view the initial reactants and the final products, the diproton lifetime is so small that they’d only see two protons fuse either scatter off of each other, or fuse into a deuteron, emitting a positron and a neutrino.
- Then that deuteron can easily combine with another proton to fuse into helium-3, a much more energetically favorable (and faster) reaction,
- And then that helium-3 can proceed in one of two ways:
- It can either fuse with a second helium-3, producing a helium-4 nucleus and two free protons,
- Or it can fuse with a pre-existing helium-4, producing beryllium-7, which decays to lithium-7, which then fuses with another proton to make beryllium-8, which itself immediately decays to two helium-4 nuclei.
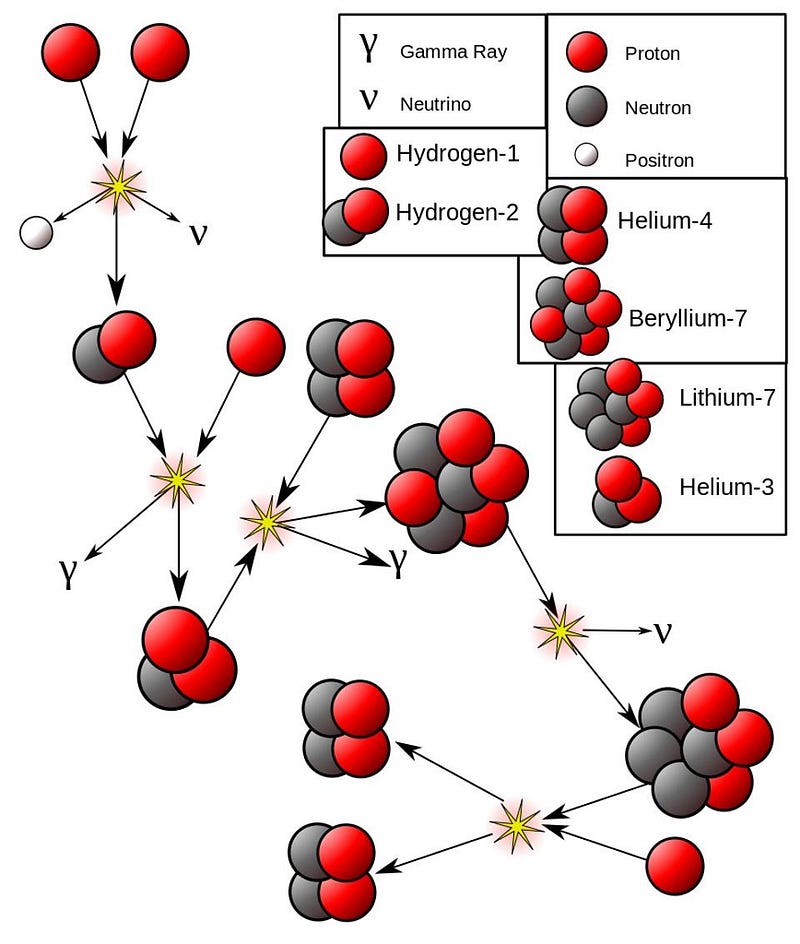
So those are the four possible overall steps available to the components that make up then entire “hydrogen fusing into helium” process in the Sun:
- Two protons (hydrogen-1) fuse together, producing deuterium (hydrogen-2) and other particles plus energy,
- Deuterium (hydrogen-2) and a proton (hydrogen-1) fuse, producing helium-3 and energy,
- Two helium-3 nuclei fuse together, producing helium-4, two protons (hydrogen-1), and energy,
- Helium-3 fuses with helium-4, producing beryllium-7, which decays and then fuses with another proton (hydrogen-1) to yield two helium-4 nuclei plus energy.
And I want you to note something very interesting, and perhaps surprising, about those four possible steps: only step #2, where deuterium and a proton fuse, producing helium-3, is technically the fusion of hydrogen into helium!
Everything else either fuses hydrogen into other forms of hydrogen, or helium into other forms of helium. Not only are those steps important and frequent, they’re moreimportant, energetically, and a greater overall percentage of the reactions than the hydrogen-into-helium reaction. In fact, if we look at our Sun, in particular, we can quantify what percentage of energy and of the number of reactions in each step is. Because the reactions are both temperature dependent and some of them (like the fusion of two helium nuclei) require multiple examples of proton-proton fusion and deuterium-proton fusion to occur, we have to be careful to account for all of them.
In our Sun, helium-3 fusing with other helium-3 nuclei produces 86% of our helium-4, while the helium-3 fusing with helium-4 through that chain reaction produces the other 14%. (Other, much hotter stars have additional pathways available to them, including the CNO cycle, but those all contribute insignificantly in our Sun.) When we take into account the energy liberated in each step, we find:
- Proton/proton fusion into deuterium accounts for 40% of the reactions by number, releasing 1.44 MeV of energy for each reaction: 10.4% of the Sun’s total energy.
- Deuterium/proton fusion into helium-3 accounts for 40% of the reactions by number, releasing 5.49 MeV of energy for each reaction: 39.5% of the Sun’s total energy.
- Helium-3/helium-3 fusion into helium-4 accounts for 17% of the reactions by number, releasing 12.86 MeV of energy for each reaction: 39.3% of the Sun’s total energy.
- And helium-3/helium-4 fusion into two helium-4s accounts for 3% of the reactions by number, releasing 19.99 MeV of energy for each reaction: 10.8% of the Sun’s total energy.
It might surprise you to learn that hydrogen-fusing-into-helium makes up less than half of all nuclear reactions in our Sun and that it’s also responsible for less than half of the energy that the Sun eventually outputs. There are strange, unearthly phenomena along the way: the diproton that usually just decays back to the original protons that made it, positrons spontaneously emitted from unstable nuclei, and in a small (but important) percentage of these reactions, a rare mass-8 nucleus, something you’ll never find naturally occurring here on Earth. But that’s the nuclear physics of where the Sun gets its energy from, and it’s so much richer than the simple fusion of hydrogen into helium!
Ethan Siegel is the author of Beyond the Galaxy and Treknology. You can pre-order his third book, currently in development: the Encyclopaedia Cosmologica.