The surprising quantum reason why the Sun shines

- Inside the Sun, enormous numbers of collisions happen between protons and other atomic nuclei in the core of our parent star.
- If we calculate how many particles should collide with enough energy to engage in nuclear reactions, overcoming their electrostatic repulsion, however, we find there are none.
- That’s where the rules of quantum mechanics come in, allowing these particles to quantum tunnel into a more stable state, enabling the fusion reactions that power our Sun.
Earth, as we know it, is only teeming with life because of the influence of our Sun. Its light and heat provides every square meter of Earth — when it’s in direct sunlight — with a constant ~1500 W of power, enough to keep our planet at a comfortable temperature for liquid water to continuously exist on its surface. Just like the hundreds of billions of stars in our galaxy amidst the trillions of galaxies in the Universe, our Sun shines continuously, varying only slightly over time.
But without quantum physics, the Sun wouldn’t shine at all. Even in the extreme conditions found in the core of a massive star like our Sun, the nuclear reactions that power it could not occur without the bizarre properties that our quantum Universe demands. Thankfully, our Universe is quantum in nature, enabling the Sun and all the other stars to shine as they do. Here’s the science of how it works.

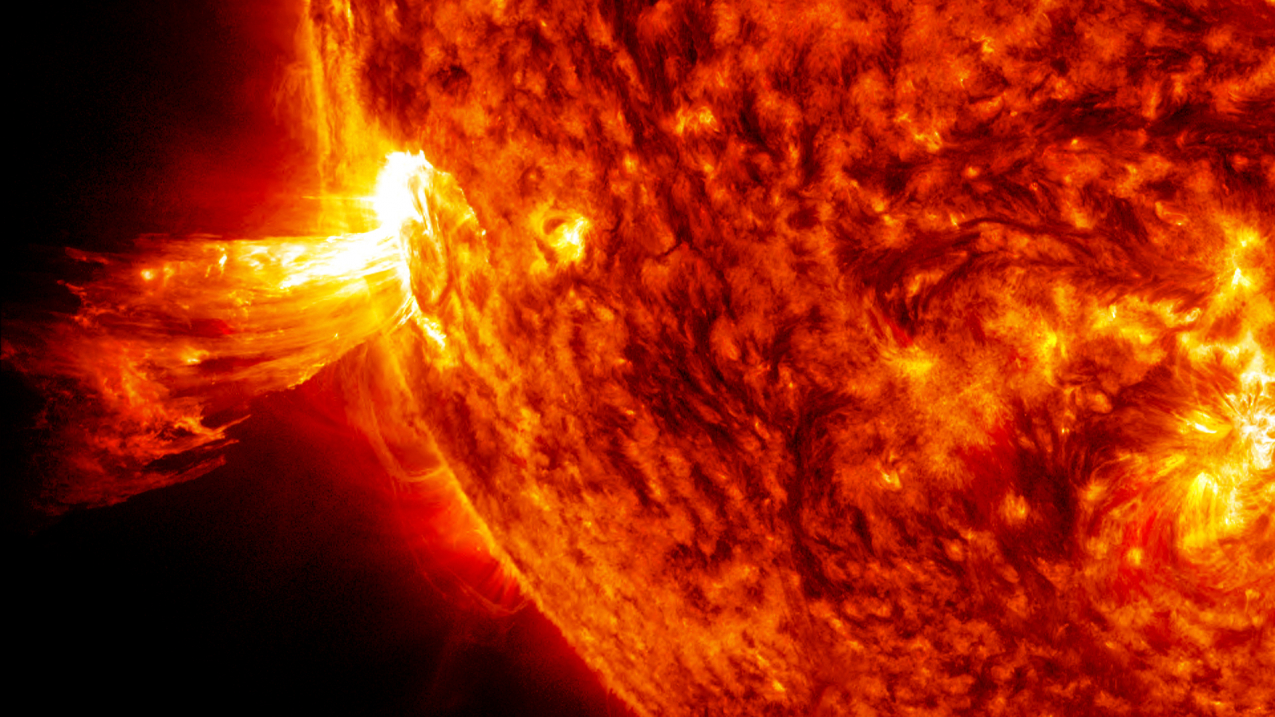
Starlight is the single greatest source of energy in the Universe throughout its entire 13.8 billion year history, subsequent to the hot Big Bang. These large, massive concentrations of hydrogen and helium contract under their own gravity when they first form, causing their cores to become denser and denser all while heating up. Eventually, a critical threshold is reached — at temperatures of ~4 million kelvin and densities exceeding that of solid lead — where nuclear fusion begins in the star’s core.
But here’s the puzzle: you can determine exactly how much energy the particles in the Sun must have, and calculate how those energies are distributed. You can calculate what types of collisions occur between protons in the Sun’s core, and compare that with how much energy is required to actually bring two protons into physical contact with one another: overcoming the electric repulsion between them.
And when you do your calculations, you find a shocking conclusion: there are zero collisions happening there with enough energy to lead to nuclear fusion. Zero. None at all.

At first glance, this would appear to make nuclear fusion — and hence, the ability of the Sun to shine — completely impossible. And yet, based on the energy we observe coming from the Sun, we know that it does, in fact, shine.
Deep inside the Sun, in the innermost regions where the temperature ranges between 4 million all the way up to 15 million kelvin, the nucleus of four initial hydrogen atoms (i.e., individual protons) will fuse together in a chain reaction, with the end result producing a helium nucleus (made of two protons and two neutrons), along with the release of a significant amount of energy.
That energy is carried away in the form of both neutrinos and photons, and while the photons might spend over 100,000 years before they make it to the Sun’s photosphere and radiate into space, the neutrinos exit the Sun in mere seconds, where we’ve been detecting them on Earth since the 1960s.
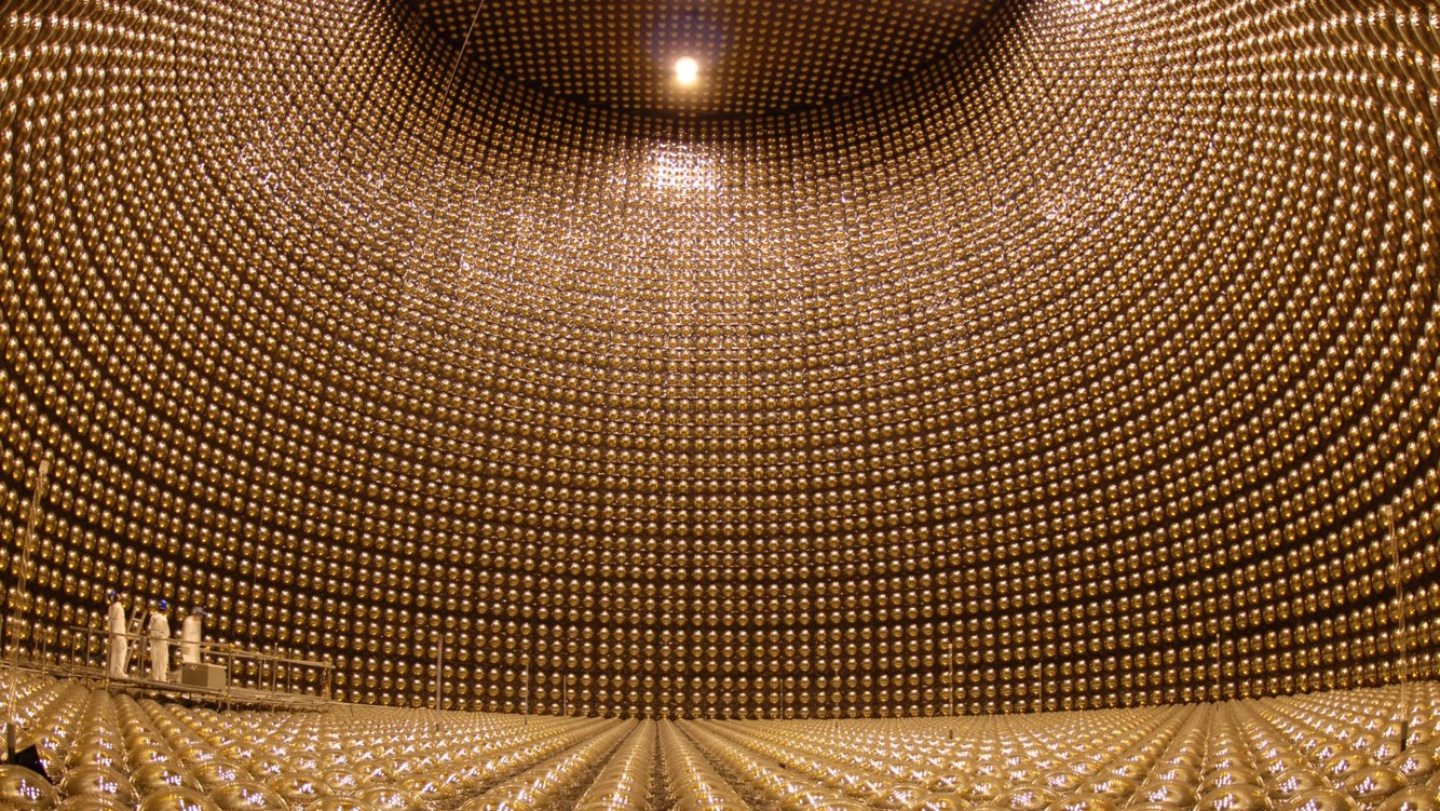
You might think about this scenario and be a bit puzzled, since it isn’t obvious how energy is released from these reactions. Neutrons, you see, are ever so slightly more massive than protons are: by about 0.1%. When you fuse four protons into a nucleus containing two protons and two neutrons, you might think that reaction would require energy instead of emitting it.
If all of those particles were free and unbound, that would be true. But when neutrons and protons are bound together into a nucleus such as helium, they wind up being bound together so tightly that they’re actually significantly less massive than their individual, unbound constituents. While two neutrons have about 2 MeV (where an MeV is one million electron-volts, a measure of energy) more energy than two protons are — via Einstein’s E = mc² — a helium nucleus is the equivalent of 28 MeV lighter than four unbound protons.
In other words, the process of nuclear fusion releases energy: about 0.7% of whatever protons fuse together gets converted into energy, carried by both neutrinos and photons.
We observe the Sun emitting, over its entire surface, a continuous power output of 4 × 10²⁶ Watts. That amount of energy translates into an enormous number of protons — somewhere upward of 10³⁸ of them — fusing together in this chain reaction every second. This is spread out over an enormous volume of space, of course, since the interior of the Sun is enormous; the average human being metabolizing their daily food produces more energy than an equivalent human-sized volume of the Sun.
But with all of those reactions occurring in the interior of the Sun, you might start to wonder how efficient these reactions are. Do we really get enough of them to generate all the power that the Sun creates? Can this really lead to such an enormous energy output, and explain how the Sun shines?
It’s a complex question, and if you start to think about it quantitatively, here are the numbers you arrive at.
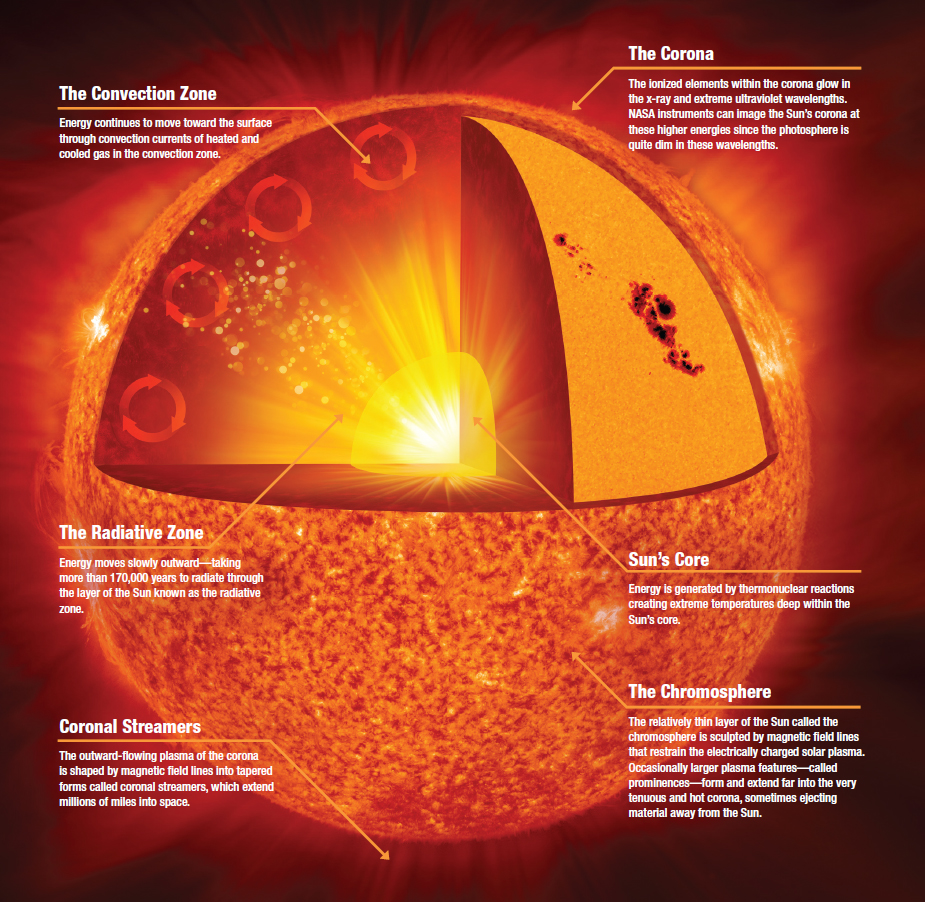
The Sun is far larger and more massive than anything we’ve experienced in our lives. If you were to take the entire planet Earth and line up a series of them across the Sun’s diameter, it would take 109 Earths to make it all the way across. If you were to take all the mass contained within planet Earth, you’d have to accumulate more than 300,000 of them to equal the mass of our Sun.
All told, there are some 10⁵⁷ particles making up the Sun, with right around 10% of those particles present in the fusion region that defines the Sun’s core. Inside the core, here’s what’s happening:
- Individual protons reach tremendous speeds, up to ~500 km/s in the Sun’s central core, where temperatures reach up to 15 million K.
- These fast-moving particles are so numerous that each proton experiences billions of collisions each second.
- And only a tiny fraction of these collisions need to create deuterium — just 1 in 10²⁸ — in a fusion reaction to produce the needed energy.

This sounds reasonable, right? Surely, given the enormous number of proton collisions that occur, how fast they’re moving, and the fact that only a tiny, almost imperceptible fraction of them would need to actually fuse, this could be achievable.
So we do the math. We calculate, based on how particles behave and move when you have a whole lot of them under a given set of energies and velocities, how many proton-proton collisions have enough energy to initiate nuclear fusion in those reactions.
To get there, all two protons have to do is get close enough to physically touch, overcoming the fact that they both have positive electric charges, and that like charges repel.
So how many of the ~10⁵⁶ protons in the Sun’s core, colliding billions of times per second, actually have enough energy to cause a fusion reaction to occur?
Exactly zero.
And yet, somehow, it happens. Not only does nuclear fusion successfully power the Sun, but stars far less massive — and with far lower core temperatures — than our own. Hydrogen gets converted to helium; fusion occurs; starlight gets created; planets become potentially habitable.
So what’s the secret?
This is the key place where quantum physics comes into play. Down at a subatomic level, atomic nuclei don’t actually behave as particles alone, but rather as waves. Sure, you can measure the physical size of a proton, but doing so makes its momentum inherently uncertain. You can also measure the momentum of a proton — essentially what we did when we calculated what its velocity is — but doing so makes its position more inherently uncertain.
Each proton, instead, is a quantum particle, where its physical location is better described by a probability function than a pinned-down position.
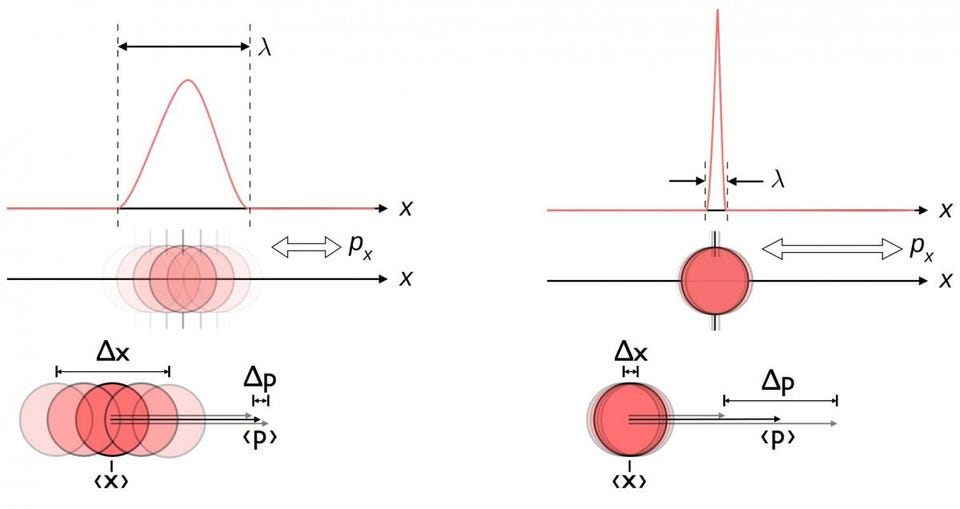
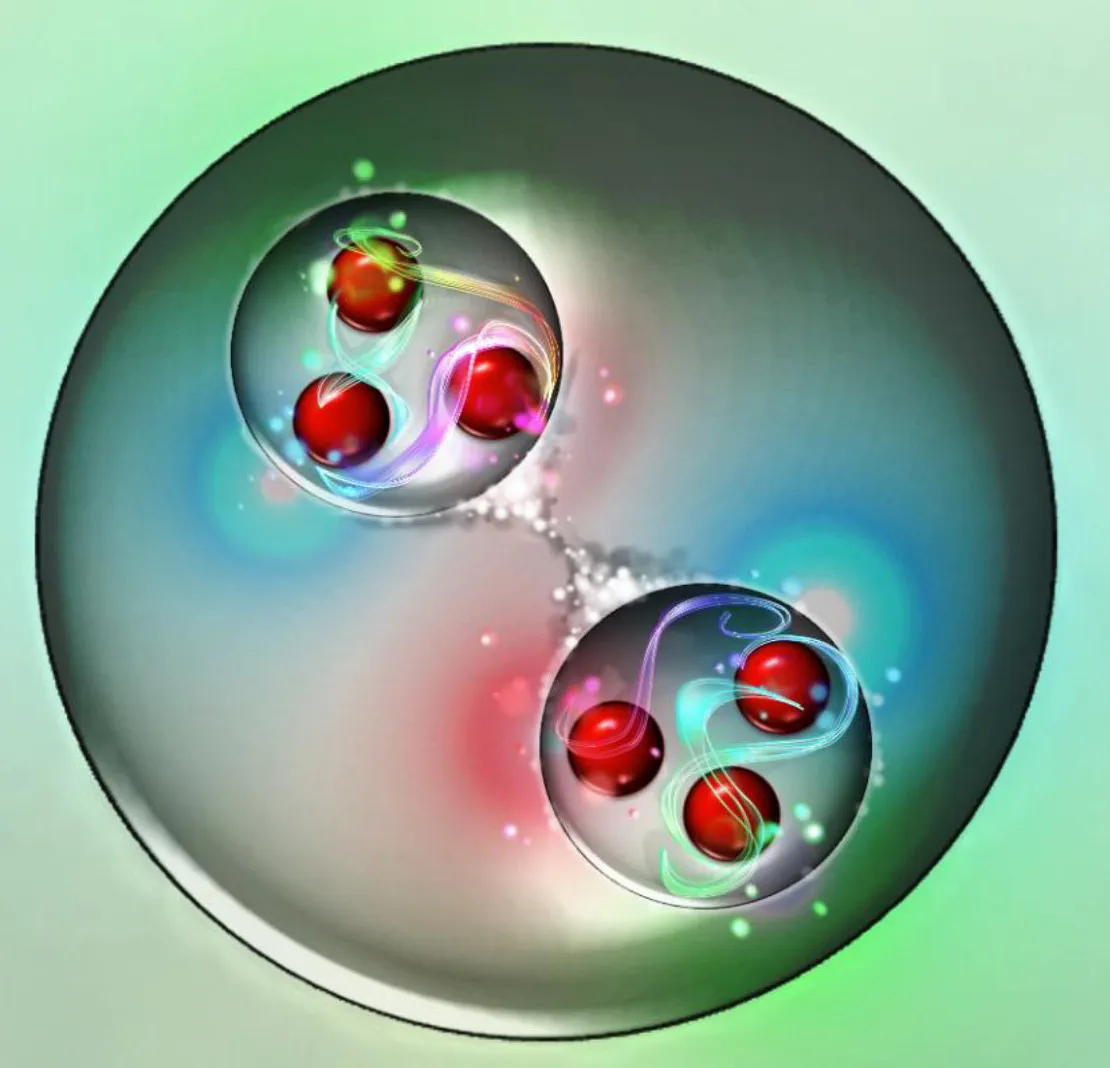
Because of the quantum nature of these protons, the wavefunctions of two protons can overlap. Even protons which don’t have enough energy to overcome the repulsive electric force between them can see their wavefunctions overlap, and that overlapping means they have a finite probability of experiencing quantum tunneling: where they can end up in a more stable bound state than their initial, free state.
Once you form deuterium from two protons — the hard part — the rest of the chain reaction can proceed quite quickly, leading to the formation of helium-4 in short order.
But the probability of forming deuterium is very small. In fact, for any particular proton-proton interaction that occurs in the Sun’s core, practically all of them will have the simplest result imaginable: their wavefunctions temporarily overlap, then they stop overlapping, and all you wind up with is two protons, the same as what you started with. But a very small fraction of the time, about 1 in every 10²⁸ collisions (remember that number from earlier?), two protons wind up fusing together, creating a deuteron, as well as a positron and a neutrino, and possibly also a photon.
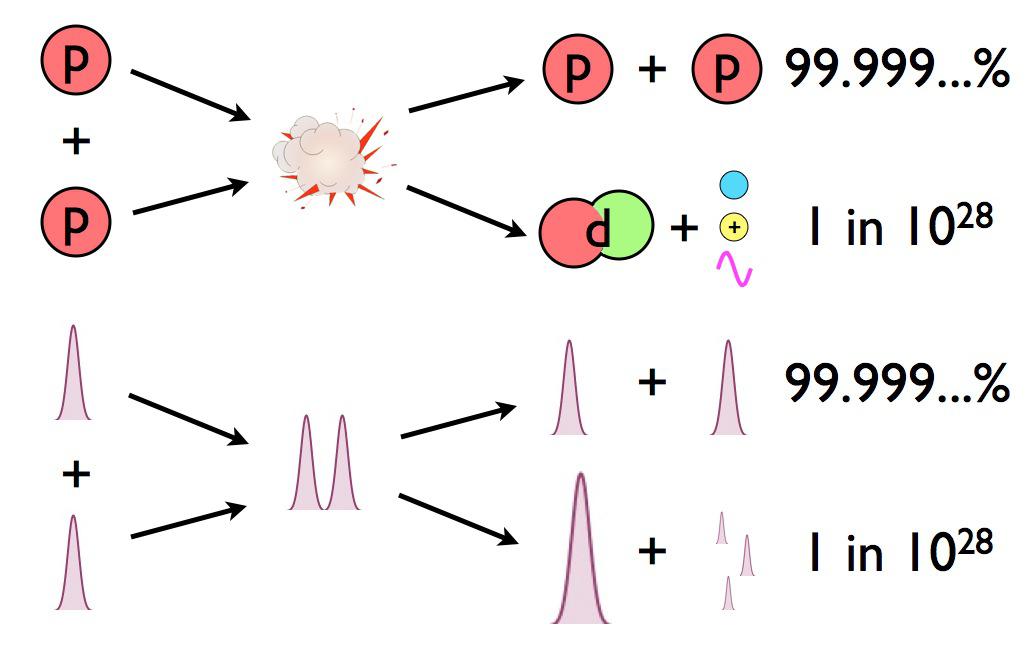
When the wavefunction of two protons in the Sun’s core overlap, there’s only a minuscule chance that they’ll do anything other than return to being two protons. The odds of them fusing together to make a deuterium nucleus are about the same as winning the Powerball lottery three times in a row: astronomically small. And yet, there are so many protons inside the Sun that this successfully occurs so often that it powers not only our Sun, but practically all of the stars in the Universe.
Over the past 4.5 billion years, this has happened enough times in our Sun that it has lost approximately the mass of Saturn due to nuclear fusion and Einstein’s most famous equation: E = mc². If it weren’t for the quantum nature of the Universe, however, nuclear fusion wouldn’t occur in the Sun at all, and Earth would simply be a cold, lifeless rock floating in the abyss of space. It’s only because of the uncertainty inherent to position, momentum, energy, and time, that our existence is possible at all. Without quantum physics, the Sun wouldn’t be able to shine. In a very real sense, we really did win the cosmic lottery.