Gold, frankincense, and myrrh: one gift was made in a neutron star

- While frankincense and myrrh were made here on Earth, gold was forged in the cosmic furnace of neutron star collisions.
- As it turns out, giant stars, supernovae, and neutron star-black hole collisions also have the capacity to make gold, but which process makes the most?
- In a new analysis, scientists quantified the various processes, and concluded that the overwhelming majority of the Universe’s gold comes from colliding neutron stars.
On a frosty winter’s night more than 2,000 years ago, a young expectant mother found herself in a wooden manger as she prepared to give birth. Shortly after the delivery, three wise men from the east arrived, bearing gifts for the newborn: gold, frankincense, and myrrh. While these three treasured gifts were all valuable, only two of them are resources unique to planet Earth. The other one — gold — is found all throughout the Solar System and the Universe. For generations, we valued this element for its rarity, shine, luster, and physical and chemical properties. What we didn’t know, however, was how to create it.
As recently as five years ago, this remained the case. While there were numerous candidate processes for how gold could be created in the Universe, we had no idea which one dominated. In fact, there were no fewer than five separate candidates for how the element gold was made:
- in the more massive stars that fuse hydrogen into helium
- in dying stars that have reached the tail end of the red giant phase
- in massive stars that undergo a supernova cataclysm
- in neutron star-neutron star collisions
- in mergers of neutron stars with black holes
Each one offered a possible pathway to create the Universe’s gold. But it wasn’t until we measured all five of them that we could determine where the overwhelming majority of gold actually comes from. The answer is neutron star-neutron star collisions, after all, and here’s how we found out.

Credit: University of Warwick/Mark Garlick
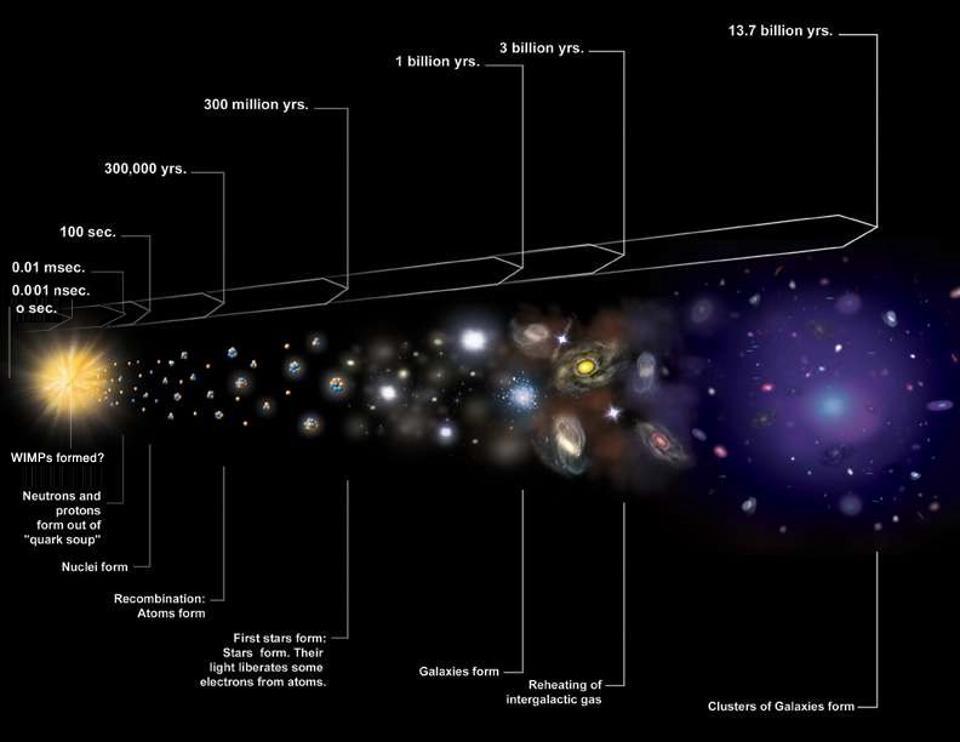
There are a slew of elements that are pretty easy to make: the ones produced by the nuclear fusion reactions that power the stars through various stages of their life. Hydrogen fuses into helium; helium fuses into carbon; carbon fuses into neon and oxygen; neon fuses into magnesium; oxygen fuses into silicon; silicon fuses into iron, nickel, and cobalt. If you want to make elements up to those last three, the basic process of nuclear fusion in stars will get you there. However, those three elements — iron, nickel, and cobalt — are the three most energetically stable nuclei in existence, with the lowest rest-mass per number of protons and neutrons in the nucleus. To build up elements beyond that — what we colloquially call “the heavy elements” — you need some other process that isn’t a result of these fusion reactions.
If you were to ask an astronomer a few decades ago where a particular heavy element on the periodic table came from, they would have told you there were three possibilities: the s-process, the r-process, and the p-process. When astrophysical objects undergo nuclear reactions, the reasoning went, you can change the composition of the atomic nucleus in one of two ways: by adding neutrons or protons to the existing nucleus. It’s a clever thought and one that’s easy to understand, even though it isn’t quite the full story.

Credit: LUNA Experiment/Gran Sasso
Here’s how those three processes work:
- The s-process is when you add neutrons steadily but slowly, increasing the mass of the nucleus until it undergoes beta decay, emitting an electron, transforming a neutron into a proton, and bumping you up one element on the periodic table. As you continue to add neutrons, in principle, you can build your way all the way up to bismuth, which has 83 protons in its nucleus. (Since gold only has 79 protons, you’d imagine that the s-process could, in principle, get you there.)
- The r-process is when you add neutrons rapidly and simultaneously. In order for this to occur, you need to bombard your nucleus with a tremendous number of neutrons all in a very short time interval, otherwise you’ll only change your elements one nucleon at a time. Whereas the slow neutron capture process adds a new neutron to a nucleus on the timescale of decades or so, the rapid neutron capture process can bombard an atomic nucleus with over 100 neutrons each second. In cataclysms like supernovae, the r-process is by far the most important one.
- The p-process, where you add protons to a nucleus, changing both your atomic mass and your atomic number all at once. Originally, the p-process referred to the creation of certain odd-numbered atomic nuclei, which were known to be neutron deficient; modern nuclear physics and nuclear astrophysics have shown us that proton capture does occur, but that it isn’t responsible for creating the elements we previously thought they did.
These processes do occur, but they aren’t everything.
Credit: NASA/CXC/M. Weiss
That’s because we now know of a few other processes that also occur. When you form elements that are heavy enough by the r-process, for example, bombarding certain nuclei with additional neutrons can trigger a nuclear fission reaction, which no doubt contributes to some of the forming elements. There’s the rp-process: the rapid proton process, which likely occurs when hydrogen, possibly from a donor star, accretes onto a compact stellar companion. And there’s also photodisintegration, where high-energy photons, in the form of gamma-rays, slam into atomic nuclei and can split them apart into smaller, lower-mass component nuclei.
Still, there are a great many unknowns. From Earth we can only do two things: Perform laboratory experiments, creating conditions to simulate the reactions that occur in cosmic environments, and observe cosmic events with the best tools available. What we’ve learned is dramatic, as we can detect the telltale signature of whether an element is present, based on the absence, or presence (and strength) of any absorption and/or emission lines. By looking in the proper part of the electromagnetic spectrum, we can determine whether any particular element was produced, and if so, in what quantity.
Credit: Sarang/Wikimedia Commons
The first stage in every star’s life is when it undergoes hydrogen fusion in its core. From the most massive blue supergiant stars to the least massive red dwarf stars, fusing hydrogen in your core is the single defining characteristic of what it takes to become a star. This is a reaction that requires core temperatures of at least 4 million K, and that means you need a mass of about 7.5% the mass of our Sun, which is about 79 times as massive as Jupiter.
There are two processes by which a star fuses hydrogen into helium, however.
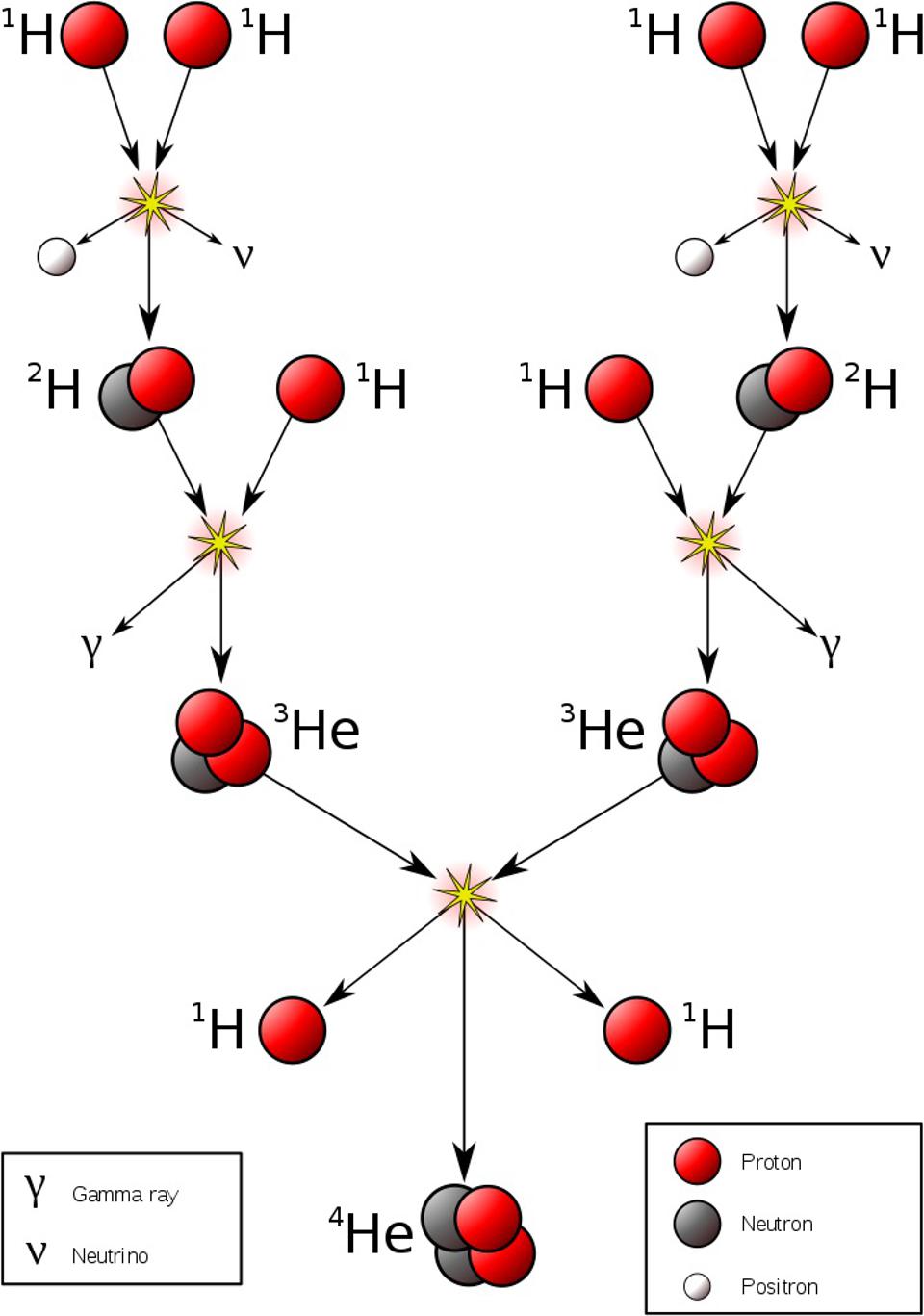
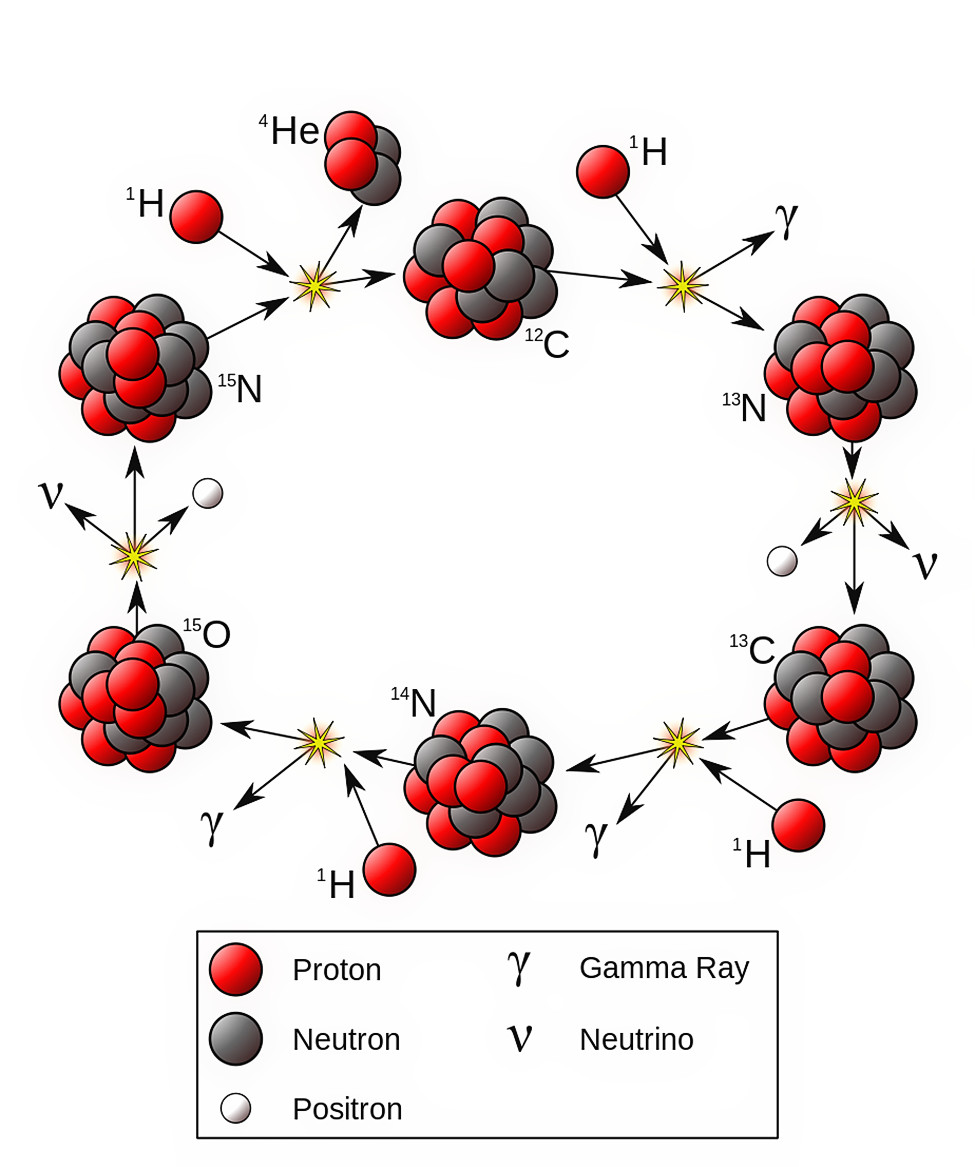
First is the proton-proton chain, which dominates at lower temperatures. Protons fuse with protons to create deuterium. Then, deuterium and another proton fuse to create helium-3. Finally, helium-3 fuses with either:
- another helium-3 nucleus, producing helium-4 and two protons
- a proton, producing helium-4 and a positron (the antimatter counterpart of an electron)
- helium-4, creating beryllium-7, which eventually gains another nucleon, becoming a mass-8 nucleus, which decays into two helium-4 nuclei
This is responsible for practically all of the nuclear fusion in red dwarf stars, and still accounts for about 99% of the nuclear fusion that occurs in our Sun.
Credit: Borb/Wikimedia Commons
The other 1%, however, becomes more important at higher temperatures and hence, at higher masses: the carbon-nitrogen-oxygen cycle. Because all stars contain carbon, except for the very first ones created immediately following the Big Bang, it’s only a question of temperature. If you’re hot enough, you’ll go through a cycle where you add protons, incrementally, to carbon, nitrogen, and oxygen, eventually leading to the emission of a helium-4 nucleus and bumping your oxygen atom back down to carbon.
Neither of these produces heavy elements (as in, heavier than iron-cobalt-nickel), but there is an important ingredient that gets created in great abundance through the C-N-O cycle and not through the proton-proton chain: carbon-13.
That’s important because later in life these stars will finish burning through the hydrogen in their cores. Without hydrogen fusion to produce radiation pressure, the star’s core cannot hold itself up against gravitational collapse. The core contracts and heats up, and once it crosses a specific temperature threshold, it can use the helium in its core to initiate a new type of fusion: helium fusion.

Credit: Chuck Magee
Although it mostly produces light and energy through the triple-alpha process, fusing three helium nuclei into a carbon nucleus, the high temperatures and the abundance of helium nuclei causes two additional reactions to occur:
- Carbon-13 can fuse with helium-4, producing oxygen-16 and a free neutron.
- Neon-22 can fuse with helium-4, producing magnesium-25 and a free neutron.
These free neutrons are vital; for the first time, the s-process can occur inside stars. Slowly but steadily, neutrons are added, allowing the elements to climb the periodic table. Yes, gold is produced in this fashion, but there’s nothing particularly special about it. You can add neutrons to platinum until it radioactively decays to make gold, but you can then add neutrons to gold until it radioactively decays to make mercury. Only when you reach lead, with 82 protons, does something special happen. Lead is stable; adding neutrons to it can cause the formation of bismuth, with 83 protons. However, adding more neutrons to bismuth creates polonium when it radioactively decays, but then unstable polonium emits a helium-4 nucleus, and we’re back down to lead. As a result, the s-process is very good for making lead, but not gold. We only get a tiny amount of our gold from this mechanism: about 6%.
Credit: Nicolle Rager Fuller/NSF
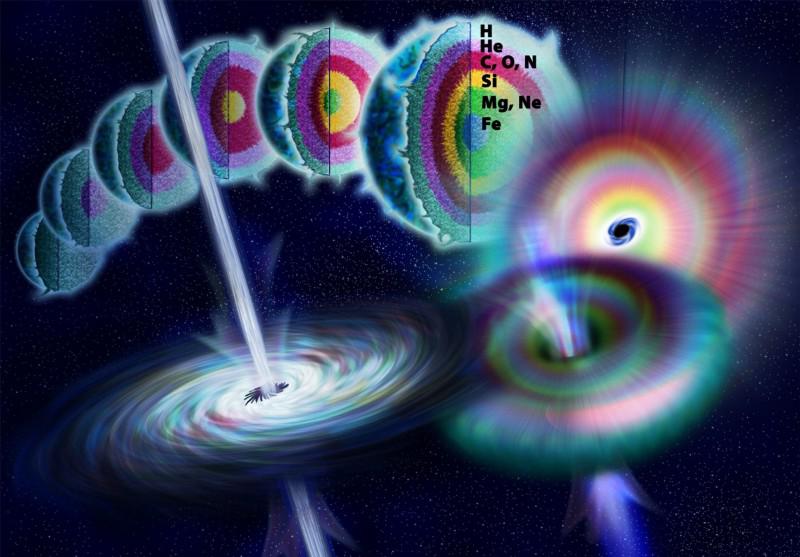
You might think to look to supernovae. With elements layered inside a pre-supernova star like an onion, with iron-cobalt-nickel at the core, surrounded by progressive layers of lighter elements, you might think that a collapsing core would produce a tremendous number of neutrons extremely rapidly. This is true, and it is the reason why supernovae are where the r-process shines.
Unfortunately for our dreams of gold, this process can build up large amounts of heavy elements, but only up to zirconium, with 40 protons. Beyond that, we just don’t see abundant elements from core-collapse supernovae. You might wonder about “the other type” of supernovae, which arise from exploding white dwarfs, but the situation is even worse there. While they also produce large numbers of neutrons and build up elements through the r-process, that doesn’t get us beyond zinc, with only 30 protons. Supernovae make heavy elements, for sure, but not the heaviest ones.

Credit: Cmglee/Wikimedia Commons
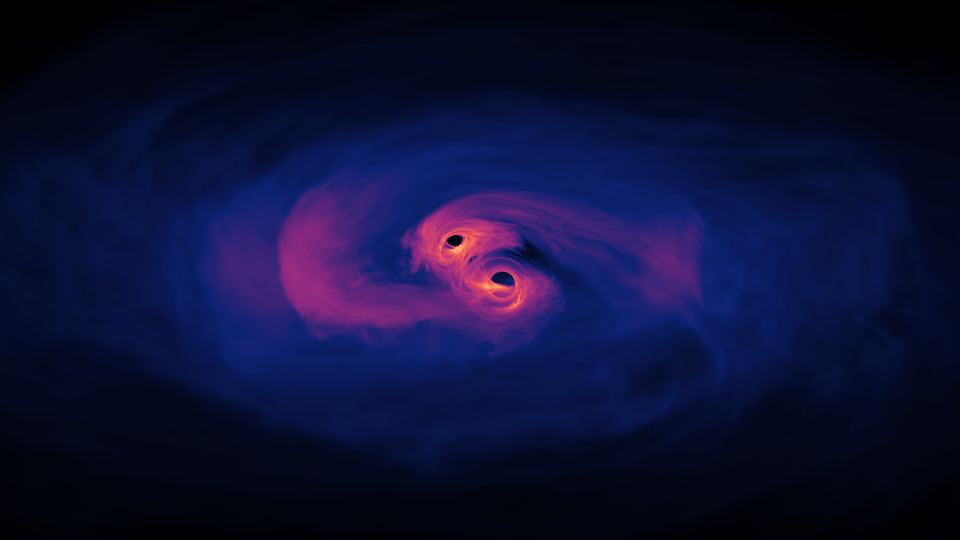
To get the majority of the heaviest elements, you need to start with what remains after a core-collapse supernova: a neutron star. Although 90% of what’s in a neutron star is — surprise — neutrons, that’s what occupies the innermost reaches of it. The outermost 10% of a neutron star is made of mostly atomic nuclei, with electrons, ions, and even atoms occupying the outskirts.
There are two ways to get a neutron star to undergo a major fusion reaction, and both of them involve causing it to interact with something else:
- Send it into another neutron star, leading to a runaway fusion reaction, a gamma-ray burst, and the expulsion of a large amount of matter. Many heavy elements are produced this way, including gold, while the cores of the merging neutron stars produce either a more massive neutron star or a black hole.
- Send it into a black hole, which will tidally disrupt the neutron star, tearing it apart. The act of tidal disruption can cause the creation of heavy elements as well, as fusion will also occur.
The fusion itself doesn’t make the heavy elements, but rather it makes copious amounts of neutrons. The r-process, among other processes like photodisintegration, rears its head again. Only this time, the targets of these neutrons are already heavy elements in both cases.

Credit: Robin Dienel/Carnegie Institution for Science
As it turns out, neutron star-neutron star mergers and neutron star-black hole interactions both produce heavy elements, and the majority of most of the heavy elements whose proton count numbers in the 40s, 50s, 60s, 70s, 80s or 90s. The copious generation of elements as light as strontium, with only 38 protons, has been observed.
But it wasn’t until October of 2021, when the results of both neutron star-neutron star mergers, like the one observed in great detail in 2017, and also black hole-neutron star mergers, were included with LIGO’s full data release. Although we haven’t detected elements directly from neutron star-black hole mergers, there are three important factors that determine the ratio of these very heavy elements that can be produced by those events:
- how large the black hole masses are,
- how large the black hole spins are,
- and how aligned the spins of the black holes and the neutron stars are.
Neutron star-black hole mergers can only produce a large fraction of those elements if there are large numbers of black holes with masses below five times the mass of the Sun, if they have large spins, and if those spins are aligned with the neutron star spins. And that’s where the gravitational wave data really allows the achievement of science to shine.
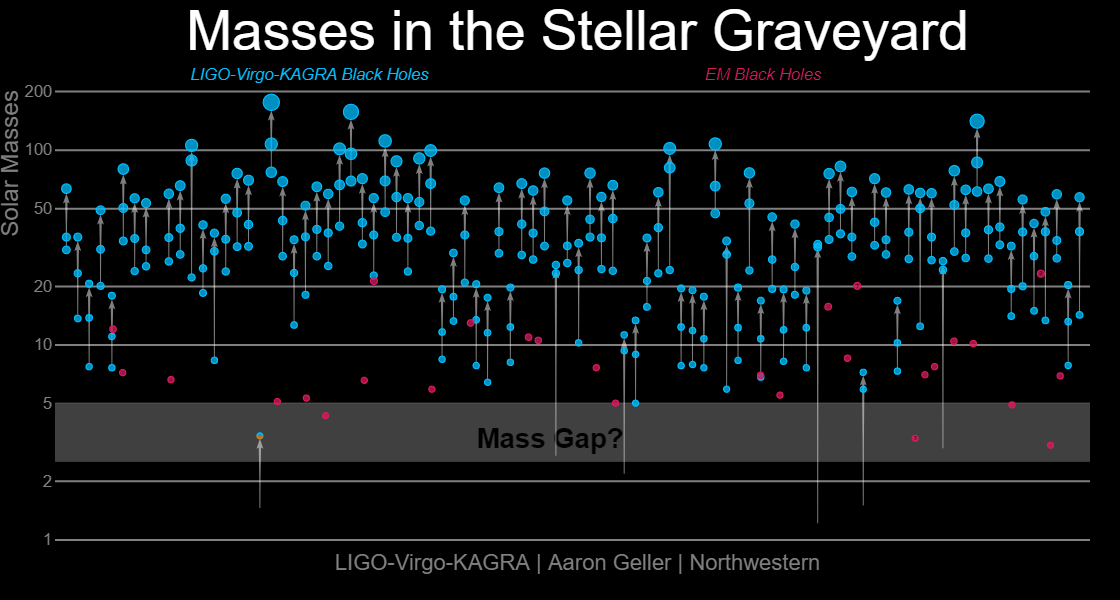
Credit: LIGO-Virgo-KAGRA / Aaron Geller / Northwestern
When all is said and done — at least, with the gravitational wave data we have so far — we’ve learned that above the threshold of the heaviest neutron stars there are far fewer black holes than you’d naively expect. Between about 2.5 and 10 solar masses there is only a small percentage of black holes, compared to the lower-mass neutron stars or the heavier black holes. The idea of a “mass gap” may be dead, but it was replaced by a cliff and a trough. There aren’t enough low-mass black holes to account for these observed elements, and moreover, the ones we’ve seen don’t have large, aligned spins when they merge with their neutron star companions.
Compared to neutron star-black hole mergers, the latest research has found that neutron star-neutron star mergers create up to 100 times the proportion of these heavy elements, and at least two-thirds of the total amount of these heavy elements overall. That includes all of the elements heavier than bismuth, but also the overwhelming majority of elements such as osmium, iridium, platinum, and gold. Whether you’re a wise man gifting it to a baby or a mirror manufacturer creating the ideal reflective surface for your infrared space telescope, gold is a rare and precious element both here on Earth and throughout the Universe. While there’s still more science to uncover, at least over the past 2.5 billion years, the overwhelming majority of gold came from merging neutron stars, and not any other astrophysical source.
This article was first published in December of 2021. It was updated in 2024.