Water in space: does it freeze or boil?

Where liquids are impossible, science gets really interesting!
“You can’t cross the sea merely by standing and staring at the water.”
–Rabindranath Tagore
If you brought liquid water into outer space, would it freeze or would it boil? The vacuum of space is awfully different from what we’re used to here on Earth. Where you stand now, surrounded by our atmosphere and relatively close to the Sun, the conditions are just right for liquid water to stably exist almost everywhere on our planet’s surface, whether it’s day or night.

But space is different in two extremely important ways: it’s cold (especially if you’re not in direct sunlight, or farther away from our star), and it’s the best pressureless vacuum we know of. While standard atmospheric pressure on Earth represents about 6 × 10²² hydrogen atoms pushing down on every square meter at Earth’s surface, and while the best terrestrial vacuum chambers can get down to about one trillionth of that, interstellar space has a pressure that’s millions or even billions of times smaller than that!

In other words, there’s an incredible drop in both temperature and pressure when it comes to the depths of outer space as compared to what we have here on Earth. And yet, that’s what makes this question all the more troublesome. You see, if you take liquid water and you place it into an environment where the temperature cools to below freezing, it will form ice crystals in very, very short order.

Well, space is really, really cold. If we talk about going to interstellar space, far away (or shadowed) from any stars, the only temperature comes from the leftover glow from the Big Bang: the Cosmic Microwave Background. The temperature of this sea of radiation is only 2.7 Kelvin, which is cold enough to freeze hydrogen solid, much less water. So, if you take water into space, it should freeze, right?

Not so fast! Because if you take liquid water and you drop the pressure in the environment around it, it boils. You might be familiar with the fact that water boils at a lower temperature at high altitudes; this is because there’s less atmosphere above you, and hence the pressure is lower. We can find an even more severe example of this effect, however, if we put liquid water in a vacuum chamber, and then rapidly evacuate the air. What happens to the water?
It boils, and it boils quite violently at that! The reason for this is that water, in its liquid phase, requires both a certain range of pressure and a certain range of temperatures. If you start with liquid water at a given fixed temperature, a low enough pressure will cause the water to immediately boil.
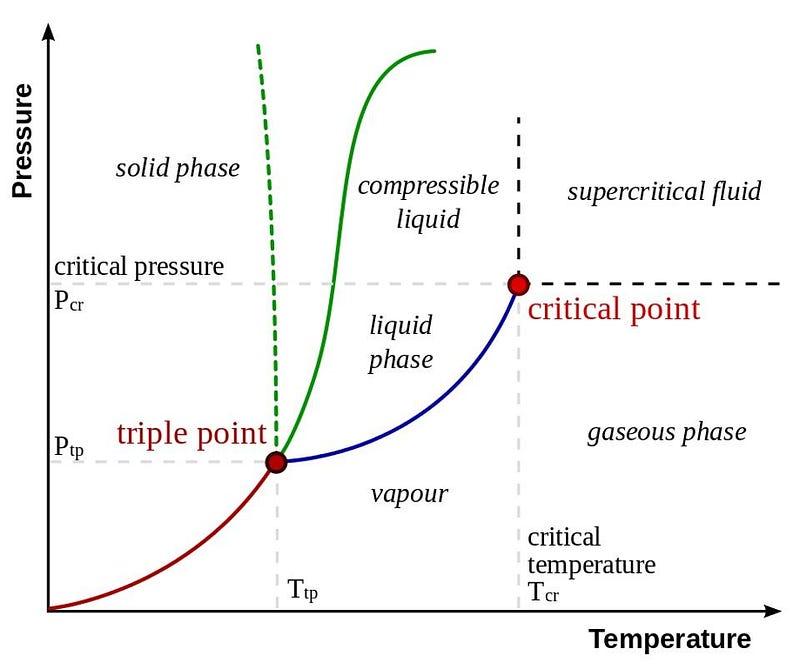
But on that first hand, again, if you start with liquid water at a given, fixed pressure, and you lower the temperature, that will cause the water to immediately freeze! When we talk about putting liquid water in the vacuum of space, we’re talking about doing both things simultaneously: taking water from a temperature/pressure combination where it’s stably a liquid and moving it to a lower pressure, something that makes it want to boil, and moving it to a lower temperature, something that makes it want to freeze.
You can bring liquid water to space (aboard, say, the international space station) where it can be kept in Earth-like conditions: at a stable temperature and pressure.
https://www.youtube.com/watch?v=ntQ7qGilqZE
But when you put liquid water in space — where it can no longer remain as a liquid — which one of these two things happens? Does it freeze or boil? The surprising answer is it does both: first it boils and then it freezes! We know this because this is what used to happen when astronauts felt the call of nature while in space. According to the astronauts who’ve seen it for themselves:
When the astronauts take a leak while on a mission and expel the result into space, it boils violently. The vapor then passes immediately into the solid state (a process known as desublimation), and you end up with a cloud of very fine crystals of frozen urine.
There’s a compelling physical reason for this: the high specific heat of water.
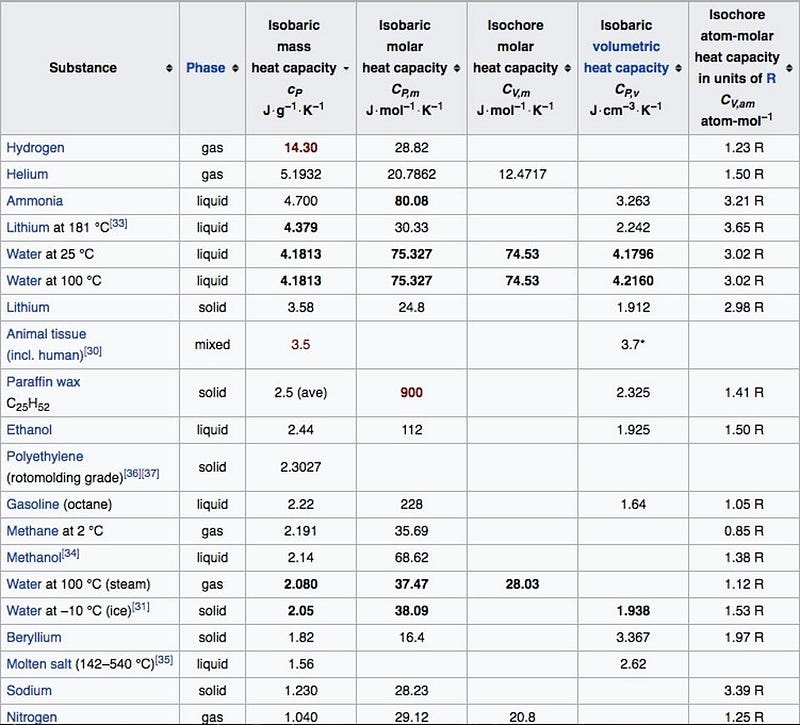
It’s incredibly difficult to change the temperature of water rapidly, because even though the temperature gradient is huge between the water and interstellar space, water holds heat incredibly well. Furthermore, because of surface tension, water tends to remain in spherical shapes in space (as you saw above), which actually minimize the amount of surface area it has to exchange heat with its subzero environment. So the freezing process would be incredibly slow, unless there were some way to expose every water molecule individually to the vacuum of space itself. But there’s no such constraint on the pressure; it’s effectively zero outside of the water, and so the boiling can take place immediately, plunging the water into its gaseous (water vapor) phase!
But when that water boils, remember how much more volume gas takes up than liquid, and how much farther apart the molecules get. This means that immediately after the water boils, this water vapor — now at effectively zero pressure — can cool very rapidly! We can see this on the phase diagram for water.

Once you get below about 210 K, you’re going to enter the solid phase for water — ice — no matter what your pressure is. So that’s what happens: first the water boils, and then the very fine mist that it boils away into freezes, giving rise to a tenuous, fine network of ice crystals. Believe it or not, we have an analogy for that here on Earth! On a very, very cold day (it has to be about -30° or lower for this to work), take a pot of some just-boiling water and throw it up (away from your face) into the air.
The quick reduction in pressure (going from having water on top of it to just air) will cause a rapid boil, and then the quick action of the extremely cold air on the water vapor will cause the formation of frozen crystals: snow!

So does water boil or freeze when you bring it to space? Yes. Yes. it does.
This post first appeared at Forbes, and is brought to you ad-free by our Patreon supporters. Comment on our forum, & buy our first book: Beyond The Galaxy!