The world is wasting our irreplaceable helium, and nobody cares
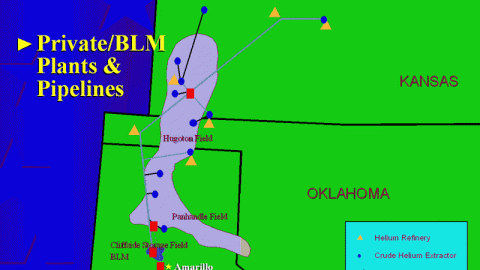
It’s not too late to change course, but we’re headed in the wrong direction faster than ever now.
“Oh Beautiful for smoggy skies, insecticided grain,
For strip-mined mountain’s majesty above the asphalt plain.
America, America, man sheds his waste on thee,
And hides the pines with billboard signs, from sea to oily sea.” –George Carlin
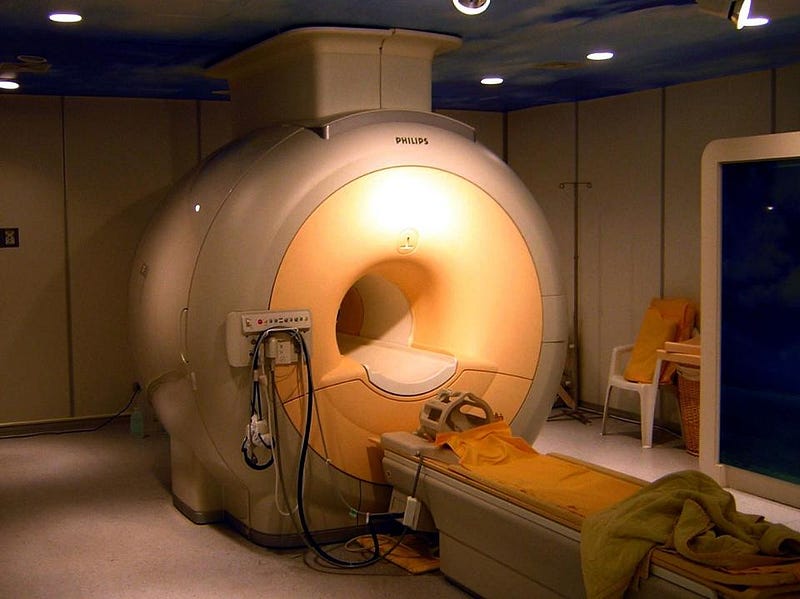
When Helium was discovered on Earth, its unique properties immediately lent itself to scientific uses. As a lighter-than-air gas, it could be used for buoyancy or even levitation. Since it’s both non-reactive and inert, it can be used at high temperatures and in oxygen-rich environments without a risk of explosion. The speed of sound is almost three times greater in helium than in air, leading to acoustic applications. And at atmospheric pressure but at low temperatures, it liquefies but never solidifies, making it the ultimate coolant for particle accelerators, MRI machines, and superconductors. Yet helium is extremely limited in abundance on Earth’s surface, and we’re making no effort to conserve it. We waste it on balloons and birthday parties, and the National Helium Reserve has been ordered to sell itself off. If we don’t do anything differently, we run the danger of exhausting the world’s supply.
Helium may be the second most abundant element in the Universe, but it’s quite a rarity on Earth. The second lightest element in the periodic table, it’s named for Helios, the ancient greek sun god, because it was discovered on the Sun, spectroscopically, before it was ever found on Earth. It wasn’t until 1882, where that same unique spectral line was seen in the lava flowing from an eruption of Mount Vesuvius. It was isolated a few years later by chemically treating igneous rocks, which separated the noble gases from the atoms they were bound together with.

But helium is too light to exist on Earth for long. Once it reaches the surface in its gaseous phase — once it comes out of the rock and makes it into the atmosphere — it’s only a matter of time before it gets kicked out into interplanetary space. Helium is lighter than all the other gases in Earth’s atmosphere, and over time it rises to the very top of the exosphere: the border between Earth’s most tenuous atoms and the vacuum of space itself. At these great heights, a strong kick from either sunlight or a solar wind particle is enough to propel a helium atom past its escape velocity, and off of Earth forever. Any large amounts of helium that Earth was formed with was ejected from our planet long ago, where the current helium fraction of our atmosphere is a meagre 0.00052%.
The way you form helium on Earth, ironically, is deep inside the planet, where the heaviest elements reside. While most of what composes Earth is stable — elements like iron, nickel, silicon, oxygen, sulphur, lead and more — there are a few notable exceptions. Elements like radium, thorium and uranium, while they might compose less than 1% of the Earth, have Einstein’s greatest equation encoded into their nuclei: E = mc2. These elements are unstable, they radioactively decay, and when they do, they convert a tiny fraction of their mass into energy via that exact rule, E = mc2.
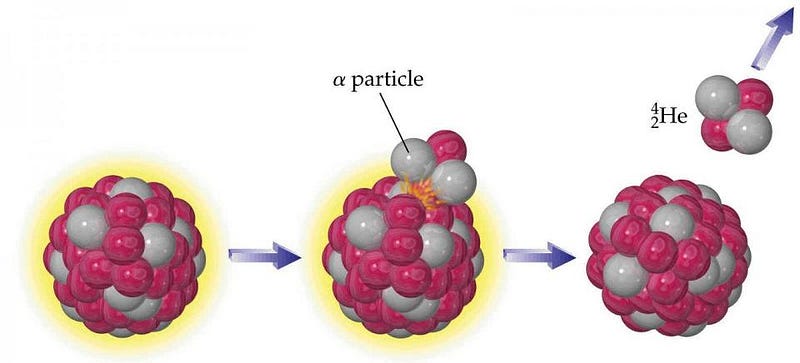
These aforementioned elements decay through a process known as α-decay, where a heavy nucleus emits two protons and two neutrons bound together, often participating in a decay chain where many α-decays happen in a row. The interesting thing is that configuration (an α-particle) of two protons and two neutrons, bound together, is also a helium nucleus! If you take a look at the planet as a whole, approximately 50% of the heat generated by the Earth itself comes from gravitational contraction, while the other 50% comes from radioactive decays. Deep within the Earth, the decay of these heavy elements means that our entire planet is a very slow helium factory.
But these elements typically have half-lives of billions of years. On the timescale of a human lifespan, the helium produced by radioactive decays is completely negligible. It takes hundreds of millions of years to produce any substantial quantities of helium underground. Once we extract it, we have to wait hundreds of millions of years again for these stores to replenish themselves. Recent exploration techniques have uncovered a few new stores of natural helium, such as the supply found in Tanzania last year, can help maintain the status quo for a little longer, but we’re feeling the crunch already.

As more and more medical and scientific applications for helium arise, the consequences of not having a large enough supply become disruptive. Helium is a finite resource on Earth, and the longer it takes us to begin conserving Earth’s stores of it in earnest, the more surely and severely we doom ourselves to a future scarcity. Certainly, there are other options for harvesting it, but they’re all astronomically expensive. Extracting helium from the atmosphere is possible — as is stealing it from other worlds — but we get a one time shot on Earth to just dig in the right spot and then contain it. Every atom we lose due to frivolity is another atom we’ll someday be forced to harvest in a much more difficult fashion.

Helium may be abundant in the Universe, but it’s rare and precious in the sense that this world only gives us a one-time shot of easily harvestable, accessible, abundant stores of it. Once we waste it, it’s gone forever, making every reachable subterranean store of helium that much more precious.
Ethan Siegel is the author of Beyond the Galaxy and Treknology. You can pre-order his third book, currently in development: the Encyclopaedia Cosmologica.