The Universe celebrates St. Patrick’s Day
There’s a chance the Earth will turn green, and even though there’s no such thing as a green star, perhaps someday, the Sun will, too.
“‘You are a different kind of Irishman, Goll,’ was all she said.
‘Every Irishman is a different kind of Irishman,’ said Goll.” –Charles Brady
When St. Patrick’s day comes around, everyone celebrates in their own particular fashion by honoring whatever Irish traditions are dearest to them. Perhaps the most universal among all the celebrations that you’ll see, of course, is the ubiquity of the color green. (In fact, you just might get yourself pinched if you’re not wearing any!)

It’d be a shame, mind you, if you didn’t realize that the planet Earth was very likely getting in on the action here, too! For those of you at very high latitudes — or, alternatively, soaring above the planet itself — there’s a uniquely green sight that’s likely to be gracing Earth’s skies: the aurorae!
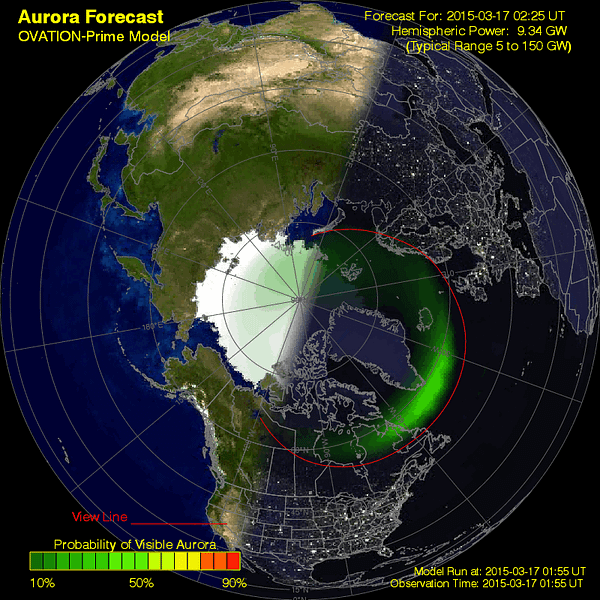
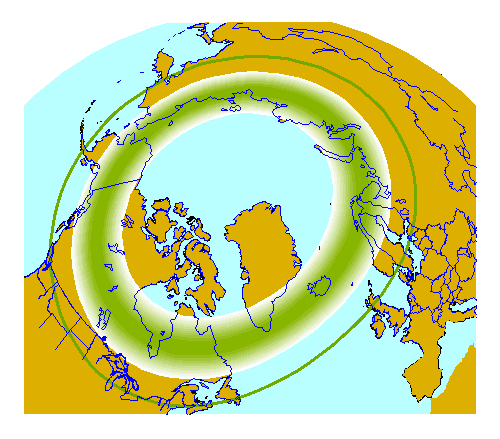
As ionized particles emitted by the Sun make their way across the 150 million kilometers of empty space between our star and Earth, our planet’s magnetic field funnels them into two circular regions focused on the Earth’s magnetic poles. The Sun just emitted a class-C solar flare recently in our direction, and so tonight — the night of March 17th — should provide prime viewing at the right locations for aurora-watchers! The charged particles from the Sun are constantly streaming in, and after approximately a three day journey, they’re bombarding the Earth, funneled into a circle by our planet’s magnetic field, and creating a chance for a spectacular show.

In fact, here is a right-now update of what it looks like, courtesy of the Aurora watcher:
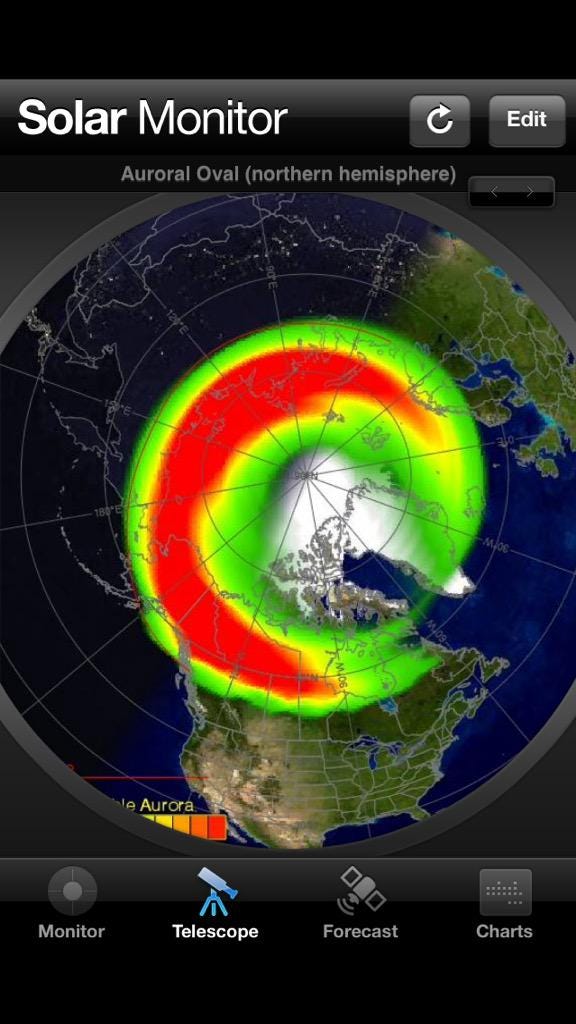
With the Moon not rising until just before dawn, the inimitable colors and sights of the aurorae should be on full display from the highest of latitudes, surrounding both poles. In fact, there’s a good chance — given clear skies — you’ll be able to see them much closer to the equator than normal! If you’re roughly at 40 degrees latitude or higher and have clear skies, be sure to look towards the poles tonight.
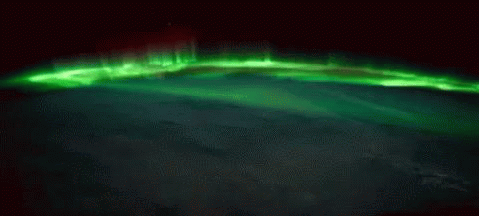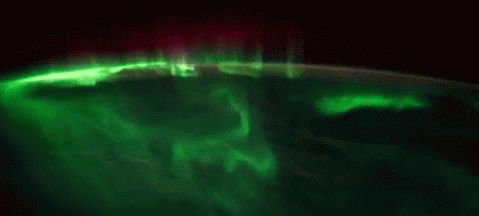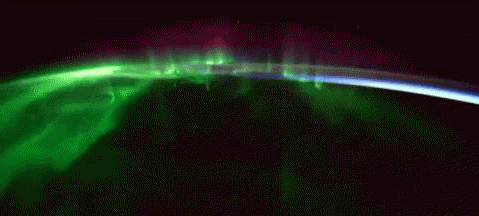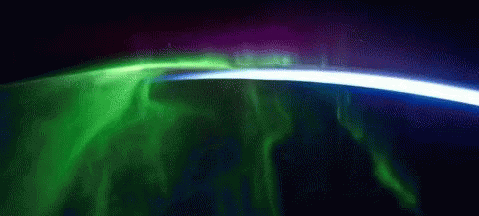
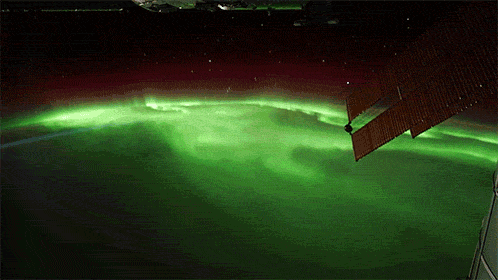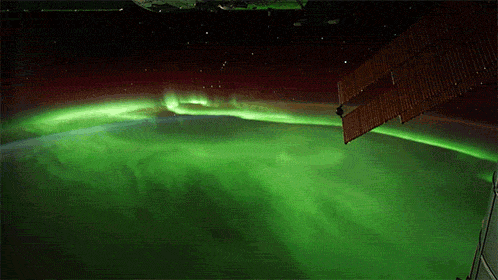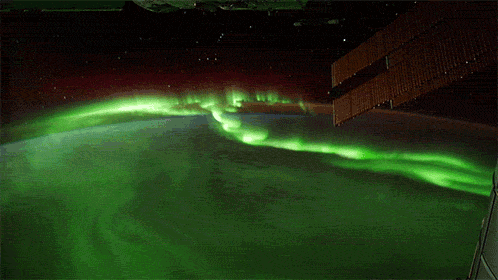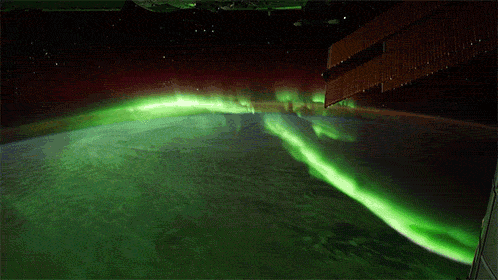
While aurorae on Earth come in a few different colors, the primary one you’ll see, the one that’s most common, brightest and most clearly visible whenever you gaze upon it, is green.
Why does the Earth’s atmosphere take on this color?

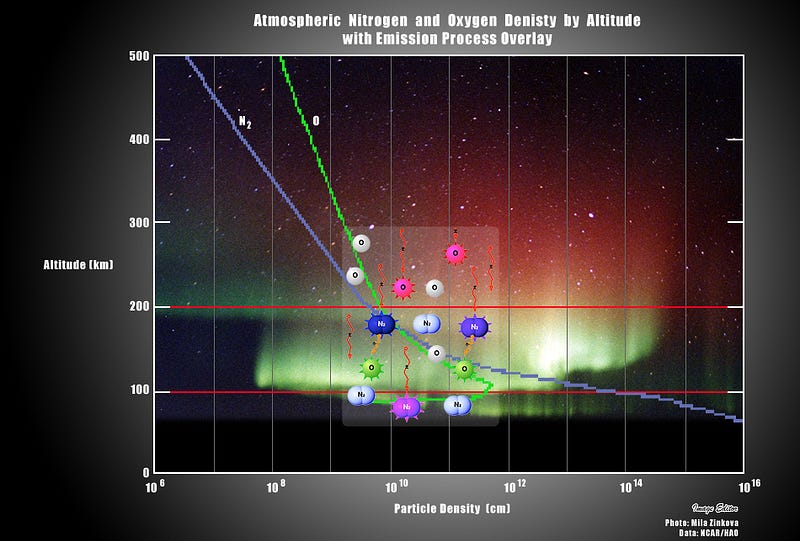
You’ve got to remember that every element in the Universe — every atom, every molecule, every configuration of nuclei and electrons — has its own unique spectrum, or sets of transitions between energy levels that electrons can take. Our atmosphere, as you well know, is made up primarily of Nitrogen (N2), Oxygen (O2), and Water Vapor (H2O), along with about 1% Argon (Ar) and only trace amounts of everything else.
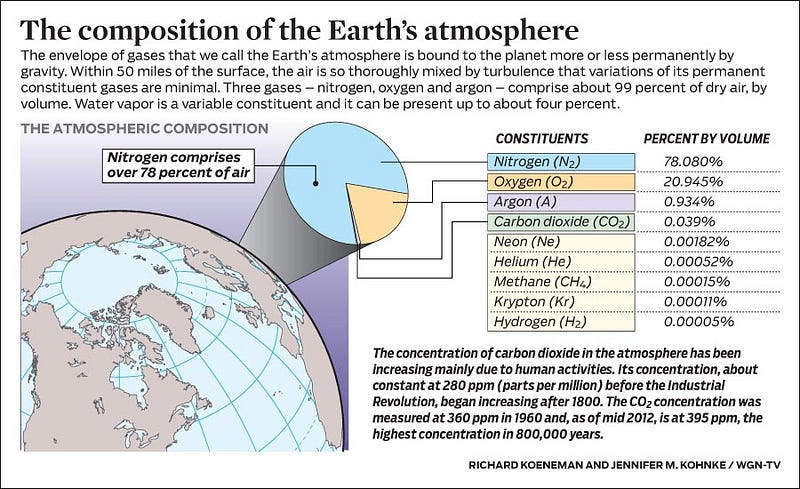
When you allow the ultraviolet light of the Sun, during the day, to shine on these molecules, they can blast them apart — temporarily — into free atoms, creating monatomic nitrogen, oxygen and hydrogen, along with ions, where some of these molecules and atoms have an electron kicked off from them.
The thing is, when night falls, these atoms, molecules and ions all encounter the electrons that were lost during the day. With no further ultraviolet radiation falling on them, the electrons can recombine with the atoms, falling down to lower energy levels and causing the emission of light. In particular, it causes the emission of light of very specific wavelengths.
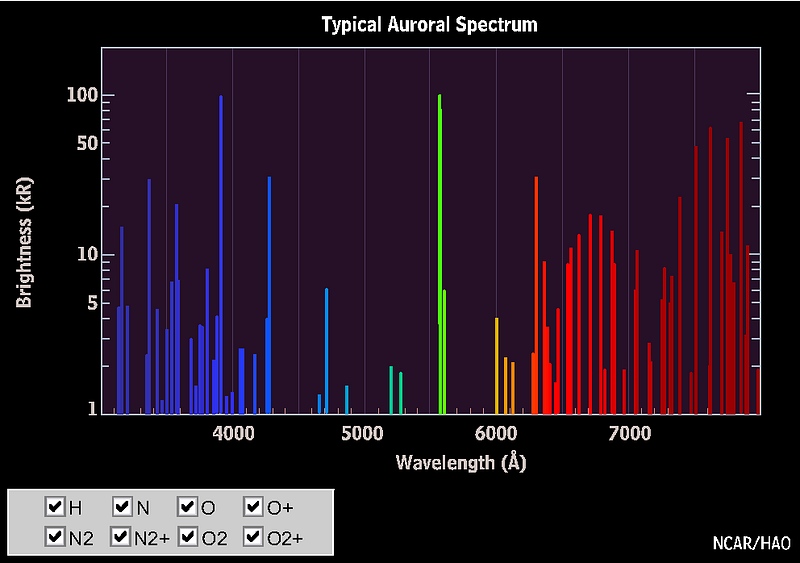
That’s normally visible as the “airglow” of Earth, but when we get bombarded by an intense solar event, the charged particles can cause these electrical excitations at night, where they become brightly visible, resulting in the aurorae, or the polar lights!

As it turns out, at about 100 km up in the atmosphere, atomic oxygen exists in abundance, and can get re-created over and over by the ionizing solar wind particles that create the aurorae. When those electrons find the oxygen atoms and settle down into the lower energy levels, they emit that characteristic green glow: an emission line at 558 nanometers.
(When the oxygen is at a higher altitude, the redder emission lines, a different set of atomic transitions, are more common, often leading to a reddish aurora instead.)
Far and away, the green color dominates the skies on most aurora-rich nights, perhaps in a poetic fashion expressing how the Earth celebrates the Irish in its upper atmosphere on a great many nights.

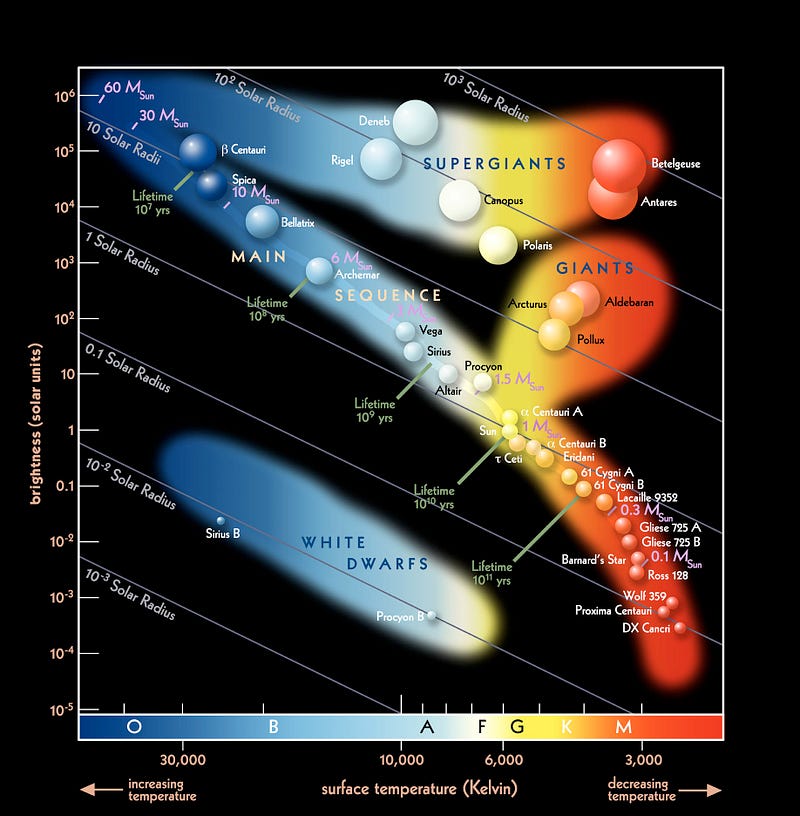
But while the Earth celebrates St. Paddy’s day in this inimitable fashion, the Sun and stars never will. A look up at the night sky, in glorious detail, shows off a plethora of star colors, ranging from red to orange to yellow to white to various shades of blue. But one of those colors — a very important one for today — is conspicuously missing!
There’s no such thing as a green star!
The reason for this is very straightforward: a star’s spectrum is an almost-perfect blackbody, meaning that the relationship of the amount of “blue” light to the amount of “red” light (the shape of its light-emission-curve) is fixed only by the star’s temperature. You can have that emission peak in the red, which it does for lower temperature stars, peak in the blue, which it does for higher temperature stars, or peak in the middle — where yellow-and-green are — like it does for Sun-like stars.
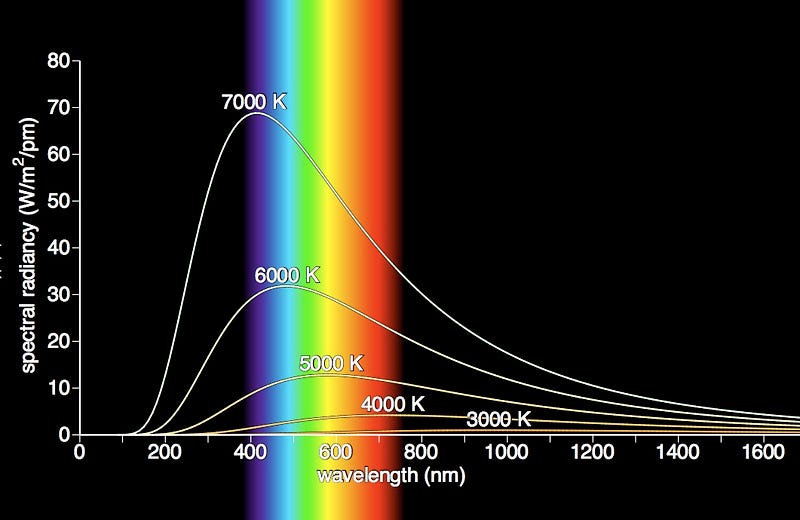
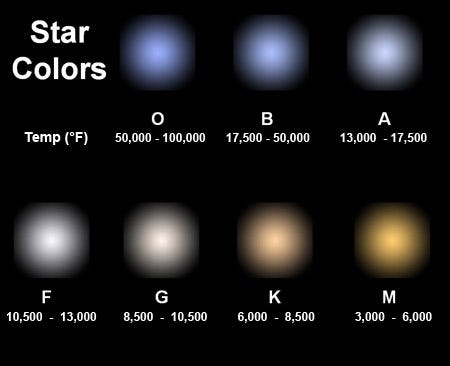
But even if your star peaks in the green portion of the spectrum, you won’t quite see it as green. Why not? The way your eyes work, you have three types of color-cones in your eyes, one specialized for blue light, one specialized for red light, and one specialized for yellow-green light. While the hottest stars have much more blue-than-red light, and the coolest stars have much more red-than-blue light, what of the stars that peak in the green?
They stimulate your red and blue cones far too much for the “green” to outshine them: you wind up seeing white, as that’s what the combination of all the colors gives you!
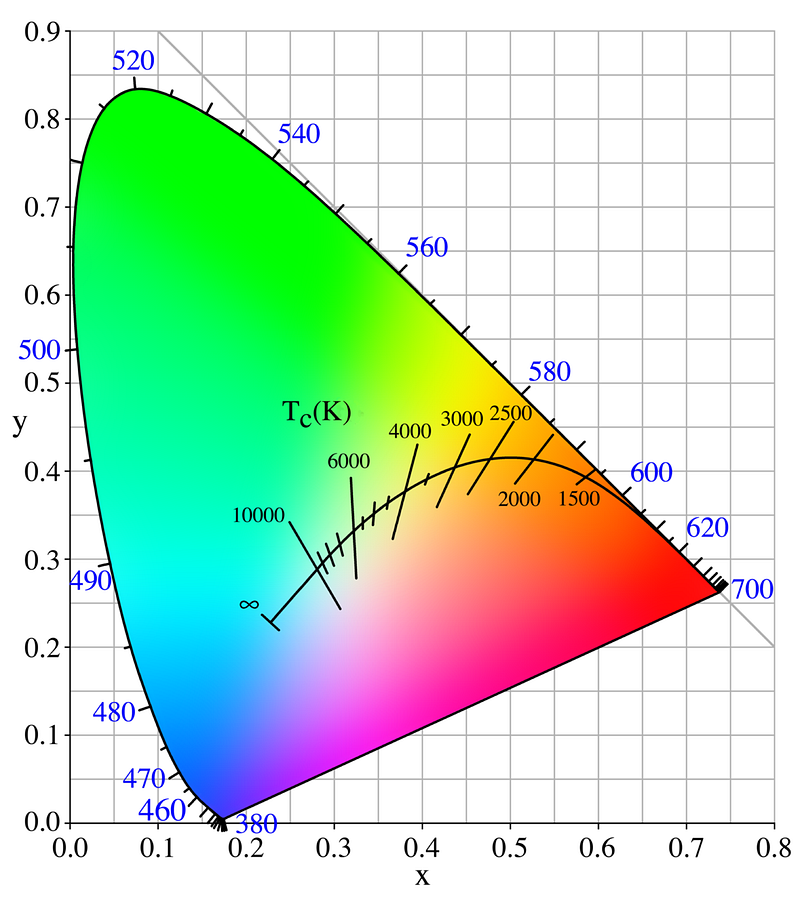
In fact, of any blackbody in the Universe — which is to say, any body that emits light because of the temperature it’s heated to — green is never a possibility. Of the entire “chromaticity” space possible, blackbodies only fall on what’s called the Planckian Locus, which is the black line shown below.
Note that while you can get colors ranging from blue to white to yellow to orange to red, you can’t get colors like purple, green or magenta.
But that doesn’t mean the color green is impossible to see in space.
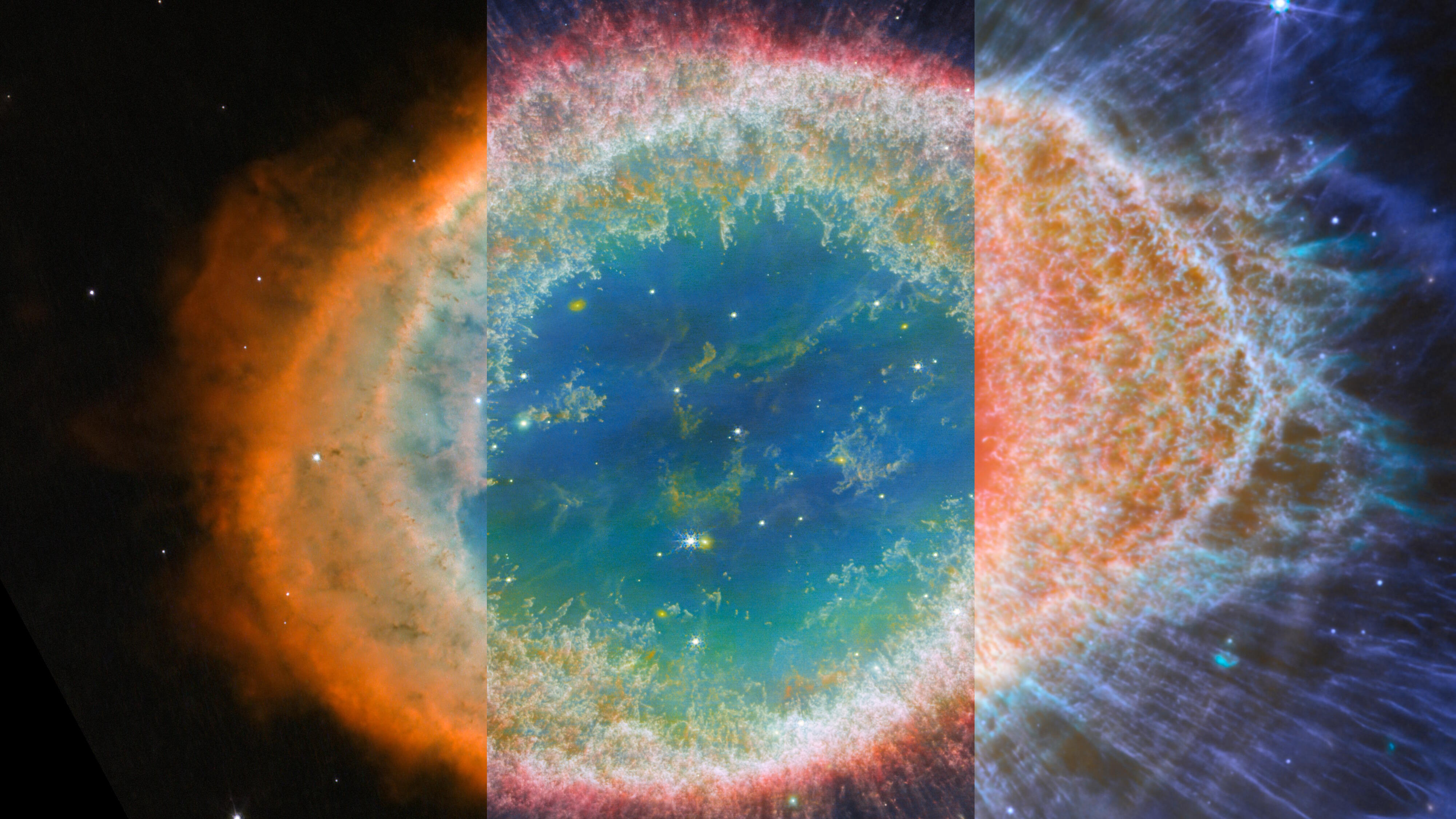
In fact, when stars like the Sun die, they swell, blow off their outer layers, and — quite often — do turn green after all!

It isn’t a green star, but rather exactly the same phenomenon we saw on Earth from our aurorae: ionized oxygen in space, whose electrons are dropping down back onto the atoms and emitting that characteristic green glow.
It’s the greatest way to celebrate St. Patrick’s Day of all: with a spectacular view of our very skies turning green, followed by a knowledge that the stars like our Sun — and possibly even the Sun itself — will one day share this rich display with the entire Universe.
Happy St. Patrick’s Day, everyone, and if you’ve got clear skies tonight, look for the aurorae near the poles!
Leave your comments at the Starts With A Bang forum over at Scienceblogs!