Scientists Didn’t Really Find The First Molecule In The Universe
There was a first molecule, and we found one just like it. But there’s a big difference.
The Universe’s first molecule is found at last! That’s what the headlines have been proclaiming this week, as NASA’s Stratospheric Observatory for Infrared Astronomy (SOFIA) has observed a hitherto elusive substance known as helium hydride. Part of it is absolutely true, as helium hydride really was the first molecule formed in the very, very early Universe, and this is the first time its presence has been detected in space, rather than synthesized in laboratories here on Earth.
But part of it’s not true. The helium hydride we’ve found doesn’t come from those early times. In fact, 100% of the helium hydride that was part of the first molecules ever made in the Universe was permanently destroyed long ago. We’ve never seen it, and most likely, we never will. Here’s why.
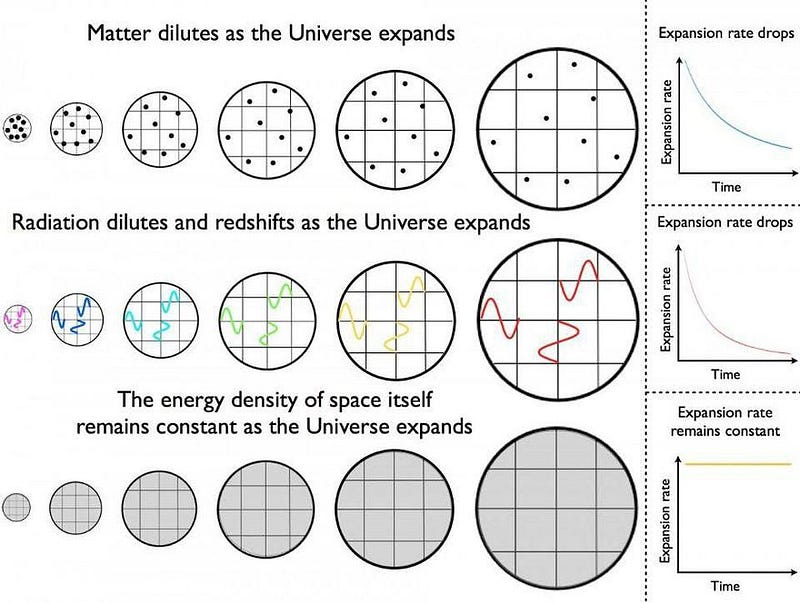
Try to imagine, if you can, the Universe as it was in the much earlier stages of the hot Big Bang. When we look at the Universe today, we see that it’s full of matter all clumped together in stars, galaxies, clusters, and along an enormous cosmic web. We see the evidence that this Universe is expanding, with distant galaxies and clusters expanding away from one another at faster rates the farther away they are. In addition, we also see the Universe filled with a bath of low-energy radiation in all directions.
This means, as time goes on, the Universe gets:
- larger,
- sparser,
- clumpier,
- and colder.
Which implies, of course, that if we look backwards in time, the opposite was true.
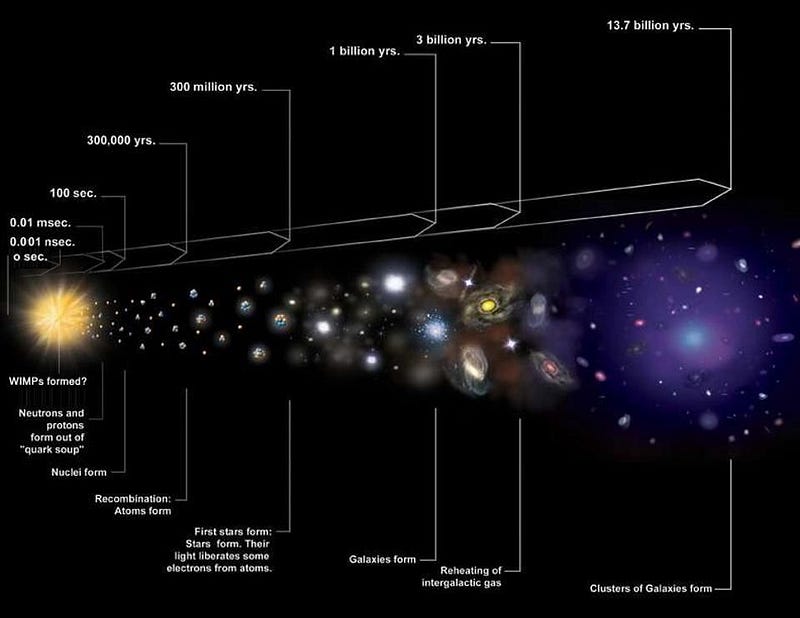
We see our Universe as it is today, some 13.8 billion years after the Big Bang. As we look farther and farther away, we’re seeing the Universe as it was when it was younger; we’re basically looking back in time. The earliest galaxies were smaller, bluer, and contained fewer heavy elements than ours do, as it’s only via the build-up of many generations of stars living and dying that we arrive at galaxies like our modern Milky Way.
In fact, we can go back to even earlier times: before we had formed any stars or galaxies. For the first few tens of millions of years after the Big Bang, gravitation hasn’t yet had enough time to work to pull the first neutral atoms together into clumps, meaning we hadn’t yet ignited nuclear fusion in them. The only fusion took place during the earliest, hottest, densest stage of the Big Bang, and gave us hydrogen, helium, and not much else.
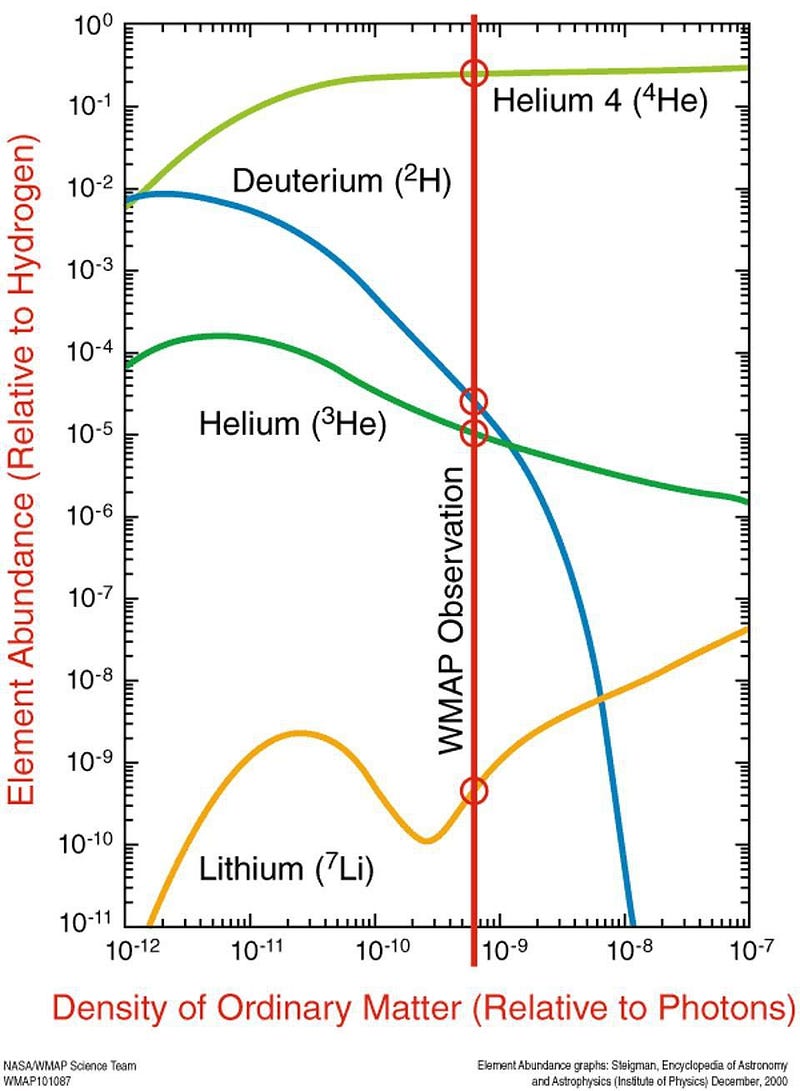
In fact, after that nuclear fusion took place during the first few minutes of our cosmic history, the Universe needed hundreds of thousands of years to cool by sufficient amounts so that we could stably form neutral atoms. Before that, the photons within it were energetic enough that they continuously knocked every single electron off of whichever atomic nucleus it happened to encounter and bind to.
When the Universe was just a few minutes old, the elements within it were (by weight) about 75% hydrogen, 25% helium, and a tiny fraction of deuterium, helium-3, and lithium. As it cooled over the millennia to come, all of the photons — including the most energetic ones that were primarily responsible for ionization — lost energy. As a result, these atomic nuclei, with different masses and different charges, begin gaining electrons at different times.
At the earliest times, everything is completely ionized, with helium and hydrogen nuclei both having no electrons at all.
After approximately 32,000 years, the Universe cools enough so that one electron can start binding to a helium nucleus. Remember, it takes two electrons to form a neutral helium atom, so helium is just halfway there at this point.
After another 100,000 years, when the Universe reaches an age of 132,000 years, that second electron can finally bind to helium without getting kicked off. We’ve got our first stable, neutral atom: helium. But helium doesn’t form bonds very easily with other atoms: it’s an inert, noble gas.
It isn’t until the Universe is around 380,000 years old that individual protons and electrons bind together to form hydrogen atoms. Hydrogen atoms can easily bond with other hydrogen atoms, producing the molecular hydrogen (H2) we’re all familiar with.
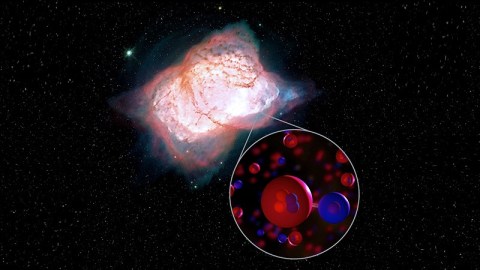
But there was an in-between time — after helium atoms form but while hydrogen is still ionized — where the first true molecules form. A molecule, remember, is simply defined wherever you have a molecular bond between one atom (or ion) and another. You might be used to molecules forming from neutral atoms binding together exclusively (like O2, oxygen), but atom-ion pairs also form molecular bonds, such as ionized carbon (C+) with neutral fluorine atoms (F), which bind together (forming CF+) and emit a photon through a process known as radiative association.
Well, when the Universe is in that in-between time, where neutral helium (He) exists but all of the hydrogen is ionized (H+), those two species can also bind together through radiative association. When a helium atom and a hydrogen ion collide, they form a molecule known as helium hydride (HeH+), and a characteristic photon is emitted which signifies the strength of the molecular bond.
Although it doesn’t appear as much in the news as physics or astronomy, the chemistry of compounds like helium hydride has a long and rich history. Helium hydride itself was discovered via its creation in the laboratory nearly a century ago: back in 1925. In theory, it should also exist in the environment of interstellar space: both in the early Universe, when it became the first molecule, but also later, when astrophysical processes create ionize hydrogen plasmas in the presence of neutral helium.

All of the early Universe’s helium hydride should have been destroyed when hydrogen became neutral, as helium hydride is far less energetically favorable than the formation of neutral hydrogen. Once you cool below a certain critical threshold, your helium hydride will interact with neutral hydrogen, preferentially forming hydrogen molecules (H2) and isolated helium atoms (He). The Universe’s first molecule didn’t last long; by the time perhaps 500,000 years passed, it was all gone.
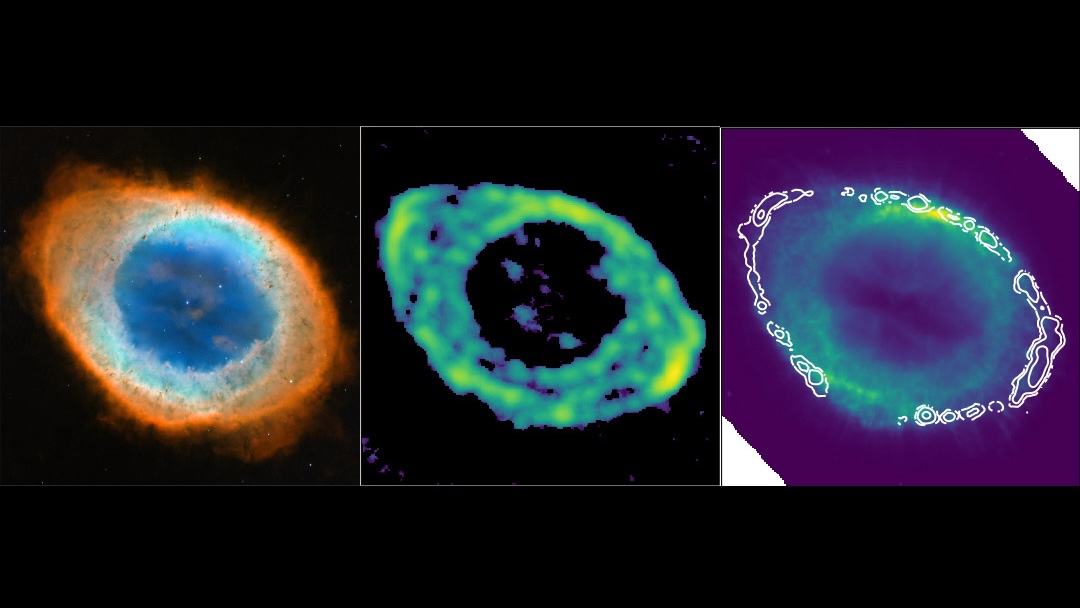
But later on, even in the modern Universe, there’s a perfect candidate location where helium hydride should exist in our Universe today: in the ionized plasmas of dying Sun-like stars. With temperatures high enough to ionize hydrogen, but plenty of neutral helium expelled from the dying stars outer layers, these planetary nebulae should be ideal homes for helium hydride.

Although it’s been more than 40 years since planetary nebulae were suggested as homes for helium hydride, observations had never caught up with it. Part of the reason is that helium hydride’s signature emissions come from a rotational transition that emits at very low energies: producing photons at 149.1 microns, placing it in the far-infrared portion of the spectrum.
You cannot see this from the ground, as the atmosphere obscures it. You can try to see it from space, but the instruments launched aboard observatories like Herschel and Spitzer were insufficient to uncover it. But that’s where NASA’s SOFIA comes in. It flies up to 45,000 feet, above the obscuring atmosphere. But, because it returns to Earth, its instruments can be easily upgraded. And upgrading the German Receiver at Terahertz Frequencies (GREAT) instrument was just what astronomers needed.
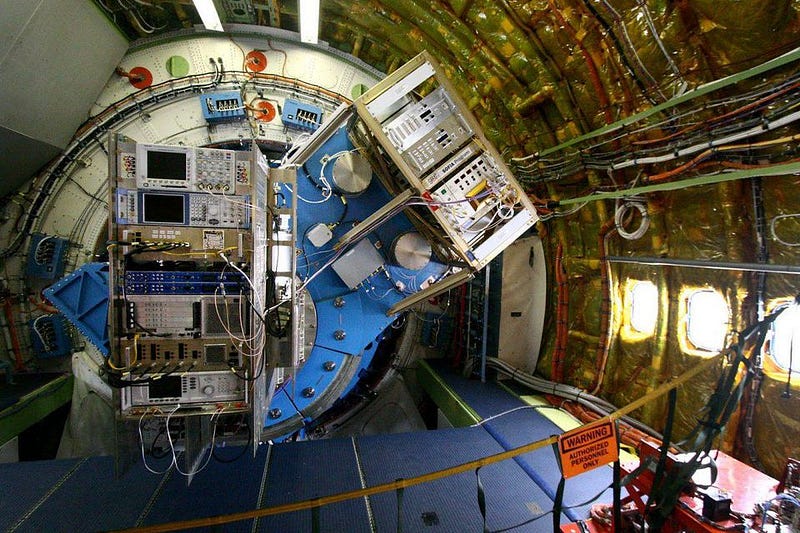
This new study determined, for the first time, that helium hydride ions really do exist in space. By observing the planetary nebula NGC 7027 with this newly upgraded instruments, scientists were able to see this characteristic transition that’s an unmistakable signature of helium hydride. According to Rolf Güsten, lead author of the new study published in Nature,
“It was so exciting to be there, seeing helium hydride for the first time in the data. This brings a long search to a happy ending and eliminates doubts about our understanding of the underlying chemistry of the early universe.”
This is the first proof we have that helium hydride can, and does, exist in the natural environment of space.

The largest lesson to learn from all of this is that there’s an incredible value to straddling the border between ground-based and space-based astronomy. Going to space is great because you no longer need to contend with the interfering effects of Earth’s atmosphere. Staying on the ground is great because you don’t have to pay for launch costs, your telescope size isn’t limited by the size of the launch vehicle, and your instruments are upgradable.
But a unique instrument like SOFIA gives us the best of both worlds. As Hal Yorke, director of the SOFIA Science Center, said,
“This molecule was lurking out there, but we needed the right instruments making observations in the right position — and SOFIA was able to do that perfectly.”
Helium hydride was long thought to be the first molecule in the Universe, but we’d never been able to detect its natural presence in space before. At long last, we have proof of its existence, and with it, further confirmation of our picture of how the Universe came to be the way it is today.
Ethan Siegel is the author of Beyond the Galaxy and Treknology. You can pre-order his third book, currently in development: the Encyclopaedia Cosmologica.