Ask Ethan: How many atoms do you share with King Tut?

The answer is larger than you might think, and applies in some shocking ways!
“The beauty of a living thing is not the atoms that go into it, but the way those atoms are put together.” –Carl Sagan
It’s no secret that when it comes to your own identity — the thing that makes you your own unique person — you’re a lot more than just the sum of the atoms that make you up. The food you eat, the water you drink, the air you breathe and everything else your body ingests can be used as raw materials for creating new molecules, new cells and new parts of your body throughout your life. The thing is, they all came from somewhere, and that’s what Brendan Markovich wants to know:
I would like to know what are the odds of the atoms in your body coming from something else in the past? Like a 0.0001% chance that somewhere in your body an atom used to be some part of a Pharaoh in Egypt or a King in England. Can science tell us anything about how atoms are recycled around the Earth and where the atoms in my body may have come from previously?
Not only can science tell you something about this, but it can allow you to estimate a whole slew of interesting facts concerning exactly what makes you up.
First off, let’s disabuse you of any incorrect preconceptions of what “you” actually are. What you think of as “you” is normally things like your bones, muscles, skin and other organs, but from a cellular standpoint, that’s only about 4% of the cells in your body. The other 96%? Split pretty evenly between blood cells and bacteria. Blood cells, most of which are red cells, live for only about 120 days apiece before they’re broken down, excreted, and replaced by new cells made in your bone marrow. Bacteria live everywhere: there are perhaps a million of them on every square inch of your skin, and tens of trillions of bacterial cells in your digestive tract.
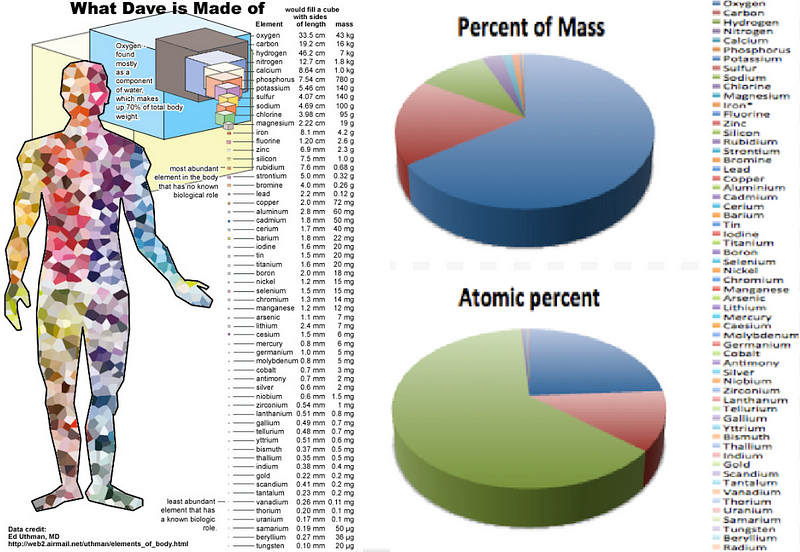
But at at even more fundamental level, these cells are made of of molecules, which in turn are made up of atoms. If we were to break the human body down into its atomic components, we’d find that there are astronomical numbers of atoms — actually, numbers that are larger than most astronomical ones — inside each one of us: predominantly oxygen, carbon, hydrogen and nitrogen.
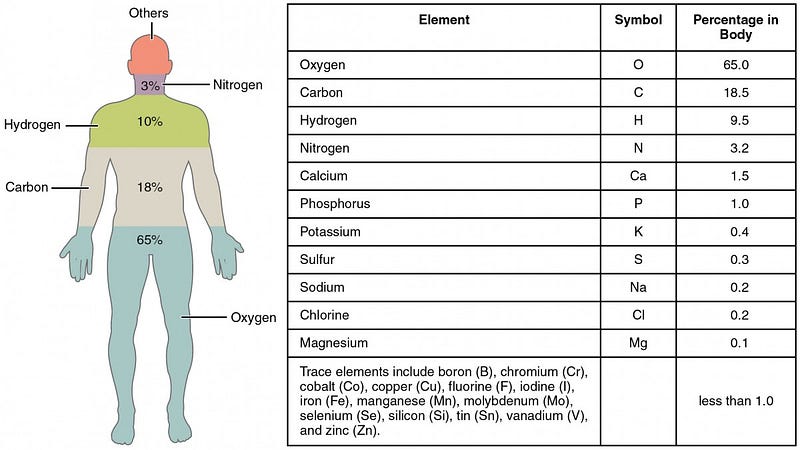
The atoms that make up each of your cells, even the calcium that makes up your bones, very rarely stay in your body for a lifetime. Instead, they’re broken down, taken into your bloodstream, filtered by your liver and kidneys, and excreted, while new atoms are ingested and built into new molecules and cells to keep you going. If you were to look at your body today versus your body 7 years ago, you’d find that over 99.999% of your atoms had been replaced! But at any given time, your body is made up of approximately:
- 1.7 × 10²⁷ oxygen atoms,
- 8.4 × 10²⁶ carbon atoms,
- 4.3 × 10²⁷ hydrogen atoms,
- 7.7 × 10²⁵ nitrogen atoms,
and less than one percent of everything else combined, led by calcium, phosphorous, sulphur, sodium, potassium, and chlorine.
Almost all of our oxygen and hydrogen comes from drinking water and breathing air, while almost all of our carbon and nitrogen comes from the food we eat. Water and air cycle relatively quickly over most of the planet, meaning that if you were looking for the air from Hitler’s last breath, Caesar’s last breath or a Tyrannosaurus’ last breath, you’d have roughly the same chances of breathing them in (assuming they breathed out the same number of atoms). But if you were looking for the carbon or nitrogen atoms that made up their bodies, those are not necessarily evenly distributed. Since oxygen and hydrogen are the most common elements in the body anyway, let’s look at them compared to two quantities: the entire atmosphere of the planet and all the water on the planet.
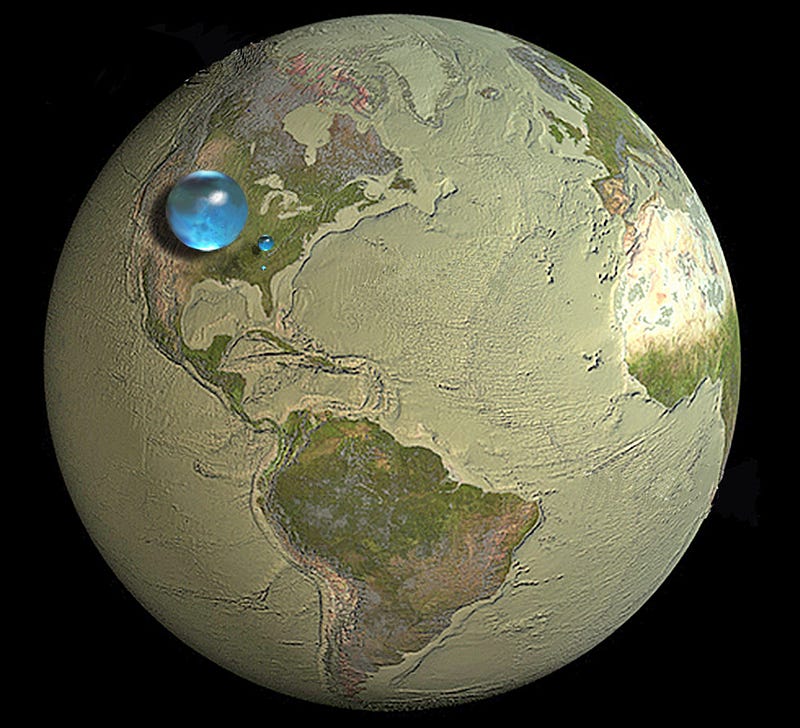
The entire atmosphere has a mass of about 5.15 × 10¹⁸ kg, meaning there are approximately 4.1 × 10⁴⁰ oxygen atoms in there (and just under four times as many nitrogen atoms); the entire sum of all the worlds lakes, oceans, seas, rivers and icecaps turns out to have a mass of 1.35 × 10²¹ kg, which gives us 4.5 × 10⁴³ oxygen atoms and 9.0 × 10⁴³ hydrogen atoms. These might seem like huge numbers, but compared to the number of atoms in a human’s body, it’s not nearly big enough.

If you were to cremate a human body, boiling off the water inside into water vapor, allowed that water to re-enter the water cycle and distribute itself evenly around the Earth, and then asked how many of those hydrogen and oxygen atoms were in a random person’s body today, you’d be floored at how large the answer is.
- One out of every 2.1 × 10¹⁶ hydrogen atoms in your body was in that random human’s body; one out of every 2.6 × 10¹⁶ oxygen atoms in your body was in that random human’s body.
- But remember, there are 4.3 × 10²⁷ hydrogen atoms and 1.7 × 10²⁷ oxygen atoms in a typical human body, like yours.
- Which means that there are approximately 2 × 10¹¹ hydrogen atoms (or 200 billion hydrogen atoms) and 6.5 × 10¹⁰ oxygen atoms (or 65 billion oxygen atoms) from any pre-existing human’s body.
That’s a lot of atoms! That means that there are hundreds of billions of King Tut’s atoms inside you right now, hundreds of billions of Hitler’s or Caesar’s atoms inside of you, and if you want to go even farther back, trillions of atoms that were a part of the Tyrannosaurus Rex, Sue, at the moment she died.

Atoms are so numerous that if we do that same style of math for the air in each of our lungs right now, we’d find that approximately one atom in everyone’s lungs, at any moment, was in Caesar’s (or Lenin’s, or George Washington’s, or Alexandre Dumas’) lungs as they exhaled their final breath. The air and water molecules get evenly distributed rather quickly and easily, meaning that you probably have a molecule or two of air in your lungs right now that was in any other living human’s lungs a decade ago. But the carbon, nitrogen, calcium and more is much less evenly distributed, so you might have millions or billions of those atoms from your historical figure of choice, or you might have none at all. (King Tut, having been mummified, would have had his water and his air returned to the larger Earth, but not his carbon or nitrogen.)
Think of this the next time you take in a deep breath or have a drink of water: you’re likely breathing or drinking in a small part of air or water that you’ve shared with every human being and most earthly creatures ever to live. At an atomic level, we’re all connected more deeply than most of us will ever realize.
Submit your Ask Ethan considerations to startswithabang at gmail dot com.
This post first appeared at Forbes. Leave your comments on our forum, check out our first book: Beyond The Galaxy, and support our Patreon campaign!