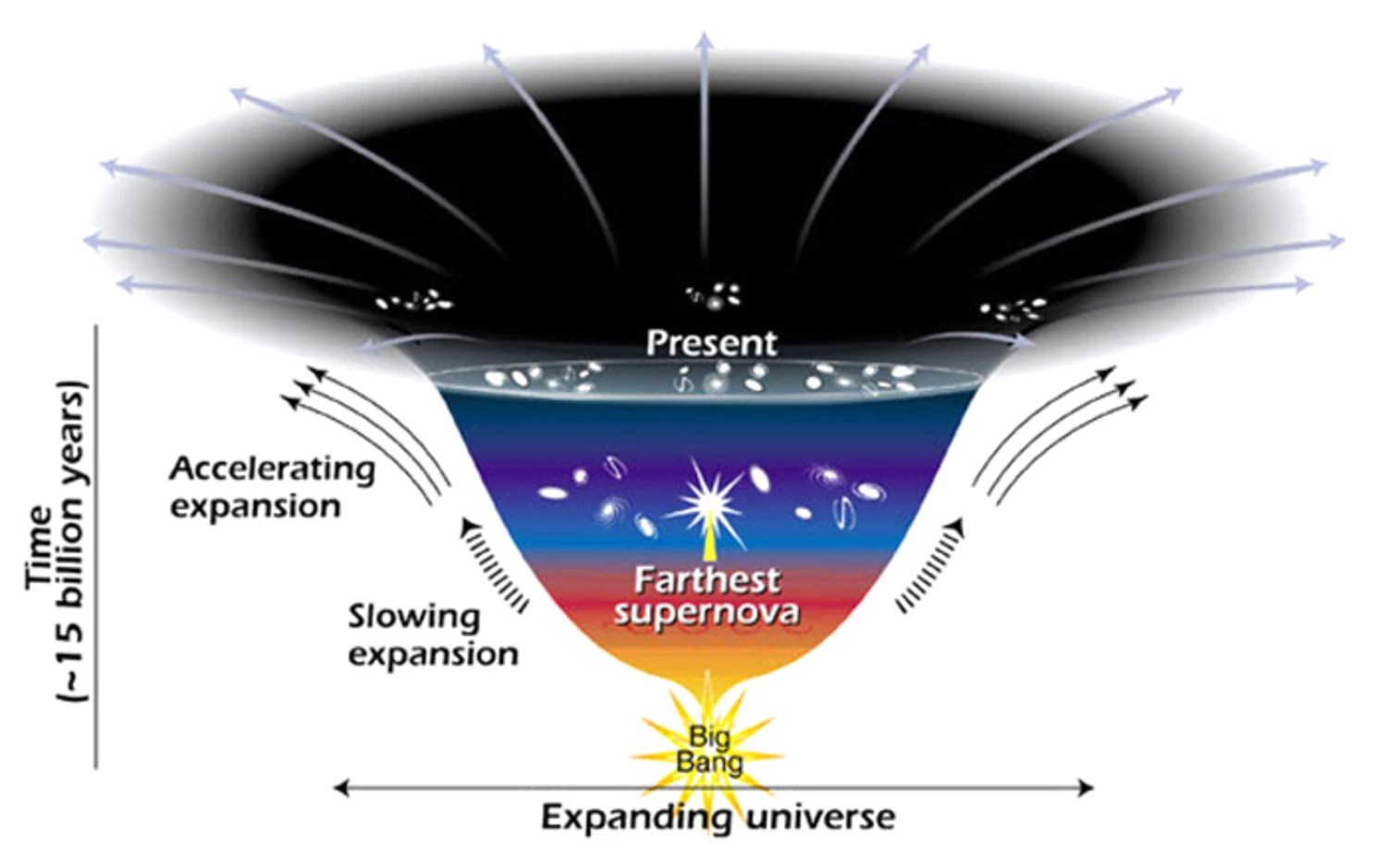
Ask Ethan: How Do We Know The Age Of The Solar System?

We’ve all heard the number: 4.5 billion years. But how do we know, and how certain are we that the Earth and Sun are the same age?
Billions of years ago, in some forgotten corner of the Milky Way, a molecular cloud like many others collapsed to form new stars. One of them formed in relative isolation, collecting material in a protoplanetary disk around it, and eventually forming our Sun, the eight planets, and the rest of our Solar System. Today, scientists proclaim that the Solar System is 4.6 billion years old, give or take a few million years. But how do we know this? And are, say, the Earth and Sun the same age? That’s what our Patreon supporter, Denier, wants to know for this week’s Ask Ethan:
How do we know the age of our solar system? […] I have a loose grasp on the concept of dating the time elapsed since a rock was liquid, but 4.5 Billion years is roughly how long ago Theia hit proto-Earth liquefying a massive amount of everything. […] How do we know we’re actually dating the solar system and not just finding dozens of ways to date the Theia collision?
It’s a great, nuanced question, but science is up to the challenge. Here’s the story.
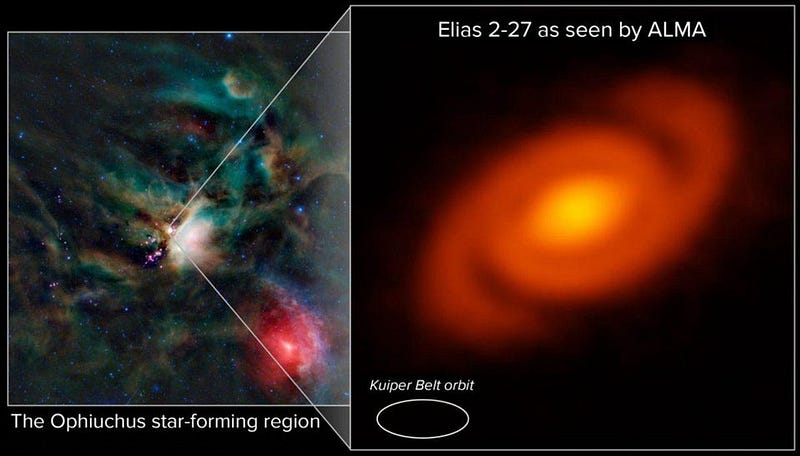
We know quite a lot about the history of our Solar System and how it came to be. There’s so much we’ve learned by watching other stars form, by examining distant star-forming regions, by measuring protoplanetary disks, by observing stars go through various stages in their life cycles, etc. But the way every system evolves is unique, and here in our own Solar System, billions of years after the Sun and planets form, all we have left are the survivors.
Initially, all stars form from a pre-solar nebula that pulls material in, with a large, outer region which remains cold, where amorphous silicates, carbon-based compounds, and ices all gather. Once the pre-solar nebula forms a proto-star and then a full-fledged star, this outer material comes in and begins forming larger clumps.
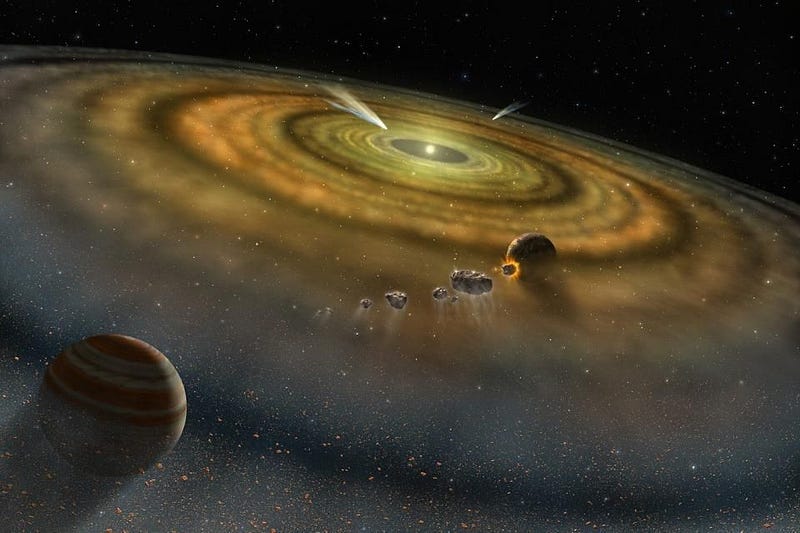
Over time, these clumps grow and fall in, where they interact, merge, migrate, and potentially eject one another. Over the timespan of hundreds-of-thousands to millions of years, once you have a star, the planets wind up forming; this is fast on a cosmic timescale. Although there were likely many intermediate objects, by time a few million years have passed, the Solar System looked pretty similar to what we have today.
But there may have been a few important differences. There might have been a fifth gas giant; the four gas giants we have may have been much closer to the Sun, having migrated outwards; and perhaps most importantly, in between Venus and Mars, there was likely not one but two worlds: a proto-Earth and a smaller, Mars-sized world named Theia. Much later, perhaps tens of millions of years after the other planets formed, Earth and Theia collided.

It was this collision that we suspect created the Moon: we call this event the giant impact hypothesis. The similarity of Moon rocks, as recovered by the Apollo mission, to Earth’s composition, has led us to suspect that the Moon formed from the Earth. The other rocky planets, which suspiciously lack large Moons, likely did not have such a large impact in their past history.
The gas giant worlds, having much more mass than the others, have been able to hold onto the hydrogen and helium (the lightest elements) that existed when the Solar System was first forming; the other worlds had the overwhelming majority of those elements blown away. With too much energy from the Sun and not enough gravity to hold onto these light elements, the Solar System began to take shape as we know it today.
But billions of years have now passed. How do we know how old the Solar System is? Is the Earth the same age as the other planets; do we have a way to tell the difference? And how what is the ultimate number for that age?
The most accurate answer, perhaps surprisingly, comes from geophysics. And that doesn’t necessarily mean “the physics of Earth,” but rather the physics of all sorts of rocks, minerals, and solid bodies. All objects like this contain a variety of the elements found in the periodic table, with different densities/compositions corresponding to where in the Solar System, radially outward from the Sun, they formed.
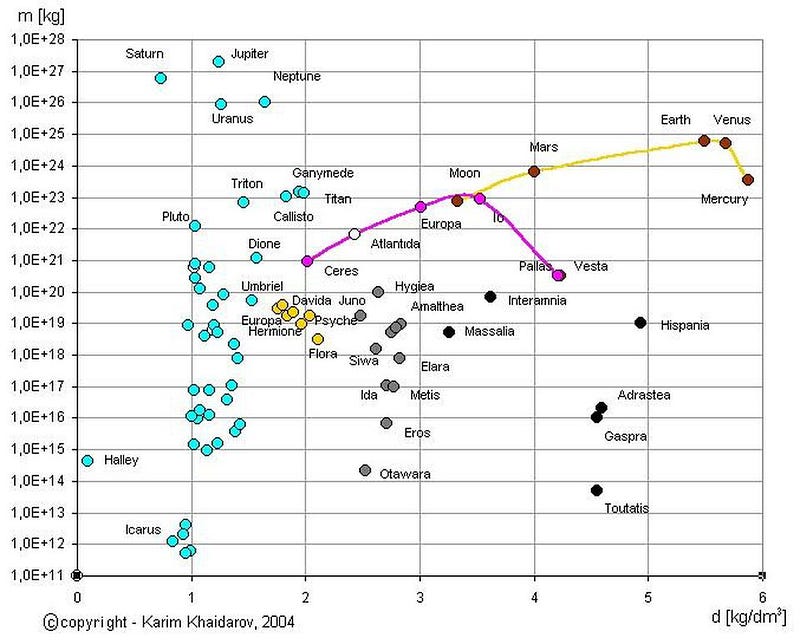
This implies that different planets, asteroids, moons, Kuiper belt objects, etc., should be preferentially made out of different elements. The heavier elements in the periodic table, for example, should preferentially be found in Mercury versus, say, Ceres, which itself should be more enriched than, say, Pluto. But what should be universal, at least you’d think so, ought to be the ratios of different isotopes of the same elements.
When the Solar System forms, it should have, for example, a specific ratio of carbon-12 to carbon-13 to carbon-14. Carbon-14 has a cosmically short half-life (of a few thousand years), so the primordial carbon-14 should all be gone. But carbon-12 and carbon-13 are both stable, meaning that wherever we find carbon in the Solar System, they should have the same isotopic ratios. This goes for all stable and unstable elements and isotopes in the Solar System.
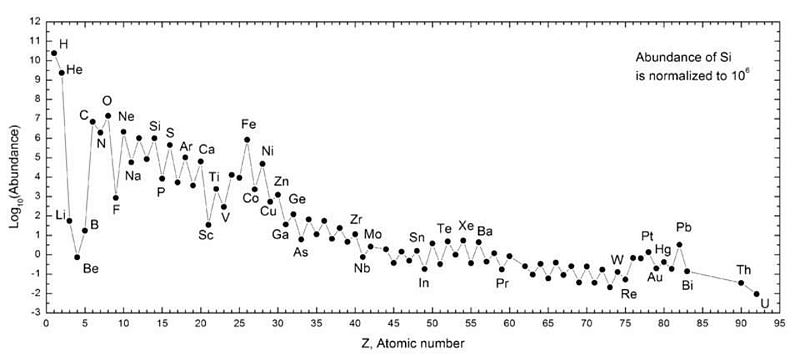
Because the Solar System is billions of years old, we can look to elements that have isotopes with half-lives that are in the billions of years. Over time, i.e., as the Solar System ages, these isotopes will radioactively decay, and by looking at the ratios of decay products versus the initial material that’s still left, we can determine how much time has passed since these objects formed. For this purpose, the most reliable elements are uranium and thorium. For uranium, its two main, naturally-occurring isotopes, U-238 and U-235, have different decay products and different decay rates, but both are in the billions of years. For thorium, radioactive Th-232 is the most useful.
What’s most remarkable, though, is that the best evidence for the age of Earth and the Solar System doesn’t come from the Earth itself!

We’ve had scores of meteorites that have landed on Earth with their isotopic, elemental abundances measured and analyzed. The key is by looking at the element lead: the ratio of Pb-207 to Pb-206 changes over time due to the decays of U-235 (which leads to Pb-207) and U-238 (which leads to Pb-206). By treating the Earth and meteorites as part of the same evolving system — with the assumption that there are the same initial isotopic ratios — we can then look at the oldest lead ores found on Earth to calculate the age of Earth, meteorites, and the Solar System.
It’s a pretty good estimate, and gives us a figure of 4.54 billion years. This is good to better than 1% accuracy, but that’s still an uncertainty of a few tens-of-million years.

But we can do better than aggregating everything together! Sure, that gives a great overall estimate, but we think that, say, Earth and the Moon are younger than the meteorites by a little bit.
- We can look at the oldest meteorites, or the ones which show the most extreme lead ratios, to try and estimate the age of the Solar System: we get a figure of around 4.568 billion years if we do that.
- We can look at the rocks from the Moon, which haven’t undergone the geological processing that Earth rocks have. They date to an age of 4.51 billion years.
And finally, we have to sanity-check ourselves. All of this was predicated on the assumption that the ratio of U-238 to U-235 was the same everywhere in the Solar System. But new evidence within the last 10 years has shown this is likely untrue.
There are places where U-235 is enriched by up to 6% over the typical value. According to Gregory Brennecka,
Since the 1950s, or even before that, no one had been able to detect any differences [in uranium ratios]. Now we’re able to measure slight differences. […] It’s kind of been a black eye for a few people in geochronology. To really say we know the age of the solar system based on the age of the rock, it’s essential that they all agree.
But two years ago, a resolution was discovered: there’s another element that plays a role. Curium, an element heavier and with a shorter half-life than even Plutonium, will radioactively decay into U-235, which explains the variations exquisitely. The uncertainties left over are only a few million years at most.
So overall, we can say that the oldest solid material we know of in the Solar System is 4.568 billion years old, with an uncertainty of perhaps just 1 million years. The Earth and Moon are perhaps ~60 million years younger, having achieved their final form somewhat later. In addition, we can’t learn this by looking at the Earth itself; the rocks that are left over here are all older than that.
But the Sun, perhaps surprisingly, may be a little bit older, since its formation should pre-date the solid objects that make up the other components of the Solar System. The Sun may be as much as tens of millions of years older than the oldest rocks in the Solar System, possibly approaching 4.6 billion years of age. The key, no matter what, is to look for the answer extra-terrestrially. Ironically, it’s the only way to accurately know our own planet’s age!
Send in your Ask Ethan questions to startswithabang at gmail dot com!
Ethan Siegel is the author of Beyond the Galaxy and Treknology. You can pre-order his third book, currently in development: the Encyclopaedia Cosmologica.