Ask Ethan #59: Double the flame, half the time?
When you throw more fuel on the fire, why does it burn out in less time?
Image credit: Wikimedia Commons user Fir0002.
“The light that burns twice as bright burns half as long — and you have burned so very, very brightly, Roy. Look at you: you’re the Prodigal Son; you’re quite a prize!” –Dr. Eldon Tyrell, Blade Runner
As the nights lengthen and winter approaches here in the northern hemisphere, many of us will be lighting candles, burning fires in our fireplaces, or setting alight fuel in our wood stoves. But if you want that fire to last longer — quite counterintuitively — you’re better off burning less of it at once! It’s the subject of this week’s Ask Ethan question, which comes courtesy of Pamela Peters, who asks:
Why, when I have a fire in the woodstove, two logs burn much faster when put in together, than one?
First off, as counterintuitive as this sounds, what Pamela notices is true.

Say you’re out somewhere (or in somewhere) and you’ve got a fire going. At least, it’s going well-enough that you can put a large log on it, and that log will start to catch fire and burn of its own accord. You can reasonably expects to get — depending on the size of your log — an hour or two (or three) out of it, as the fire slowly eats its way in, consuming the fuel of the wood as it goes.
But what if you put two (or more) similarly-sized logs on that same fire?

The flames will burn brighter, the wood (and fire) will burn hotter and faster, and — despite having more fuel at your disposal — the logs will be nothing but ash in a much shorter amount of time.
This phenomenon is something that many of you, as a child, with a few candles at your disposal and a mild predisposition towards pyromania may have noticed. (Just me? Nah, couldn’t be just me!)


If you have a single candle lit on its own (or two well-separated candles burning separately), you have a series of simple, self-sustaining chemical reactions catalyzed by heat. Let’s take a look at what they are, in four steps.
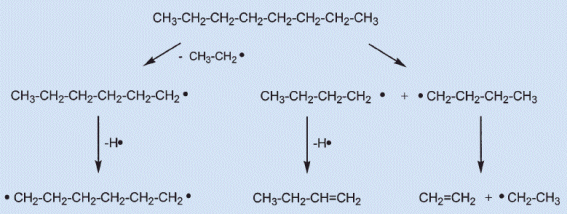
1.) First, the hydrocarbon-based fuel, molecular chains of carbon atoms (with hydrogen atoms attached) bonded to other carbon atoms are broken apart into smaller chains and, eventually, dimers and monomers. This process actually absorbs energy (is endothermic), which is why, counterintuitively, the temperature right at the fuel source is not the hottest part of a fire!
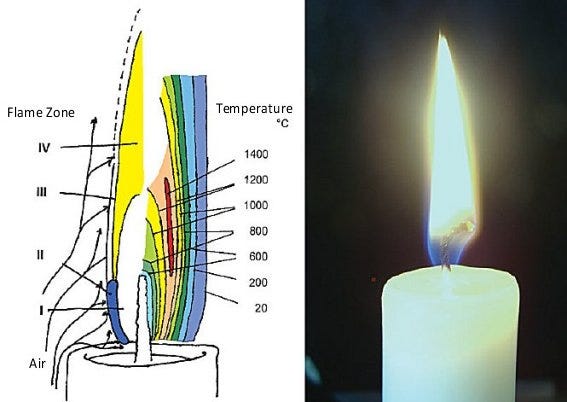
2.) Next, these small chains, moving away from the fuel source and towards higher temperatures, encounter oxygen molecules, which are highly reactive. The reaction is simple: a hydrocarbon combines with oxygen to produce water and carbon dioxide as end products, with some carbon monoxide and free radicals produced as intermediaries. (The intermediaries, by the way, don’t always burn to completion.) This process gives off energy (is exothermic), which means the locations where this reaction takes place most efficiently result in the brightest and hottest part of the flame.


3.) And finally — and this is the part that’s important for the flame you see — soot is produced. You might have thought, as most people do, that the bright yellow flames you see is simply a result of hot, ionized plasma glowing. Not quite! Soot molecules can be quite complex, consisting of more than a million atoms in many cases. If you expose them to a hot enough temperature, and we’re talking temperatures of 1,200 °C or above, they’ll begin to convert that thermal, heat energy into visible light, and that visible light peaks at yellow wavelengths. You’ll notice, by the way, that if you shine bright enough light on a flame to produce a shadow (top right), you’ll see one where the flame is; that’s due to the soot!
The reason there isn’t any soot rising above it is that — in the presence of oxygen and at temperatures over 1,000 °C — the soot then burns to completion. Once you exit the oxygen-poor region around the flame, combustion ensues once again, and you cannot see the soot. Only if you divert the soot (top left) to lower temperatures can you see it, a brilliant experiment devised by Faraday in the 1800s!
So that’s how it works for a single flame source of pretty much any type. So why would either merging two candle flames together or adding additional logs onto a fire cause the process to go faster?

Because the limiting factor to how quickly a fire burns — which is, remember, the reaction rate for combustion — isn’t typically the amount of fuel available, nor is it the amount of oxygen available. Instead, it’s the volume of that region of space that has sufficient energy/temperature for combustion to occur, and how quickly that combustion proceeds in that region.
And this is a self-sustaining process, remember: the faster combustion occurs, the higher the temperatures achieved, and therefore the more efficiently and more quickly further reactions proceed! So if you place two candles together, the combined flames achieve a higher temperature, burn the fuel more quickly and cause you to burn through your fuel significantly faster than you would separately. If you place twice as many logs on the fire (and aren’t limited by oxygen), you’ll achieve higher temperatures and increase the reaction rate of burning the fuel in the wood, burning through the entire supply more rapidly. And if you throw twice as many coals in your coal-fired engine, your engine will produce more than twice as much power, but will run out of fuel more quickly.

This is why, in the midst of a raging wildfire, as much as tens of thousands of acres of forested land can be completely destroyed in a matter of days. Increase the temperatures and the reaction rate, and your reaction proceeds to completion even more quickly.
It isn’t only chemical reactions, mind you, like fire, that are subject to this same effect. In any reaction where energy is a catalyst for your self-sustaining reaction, either adding more energy or bringing additional amounts of reactive material in will help that reaction proceed to completion more quickly. Including in stars!
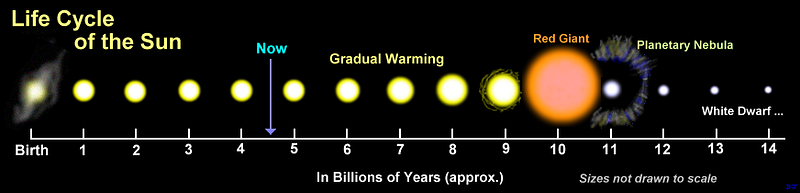
A G-type star like our Sun achieves temperatures of 15 million Kelvin at its core, and will burn through all its nuclear fuel in about 12 billion years. But a star with as little as 8% of our Sun’s mass — an M-type star — will still undergo nuclear fusion in its core at temperatures of only 4 million Kelvin. But those stars will take more than 1,000 times as long as our Sun to burn through their fuel, even though they only have 8% of the Sun’s fuel!
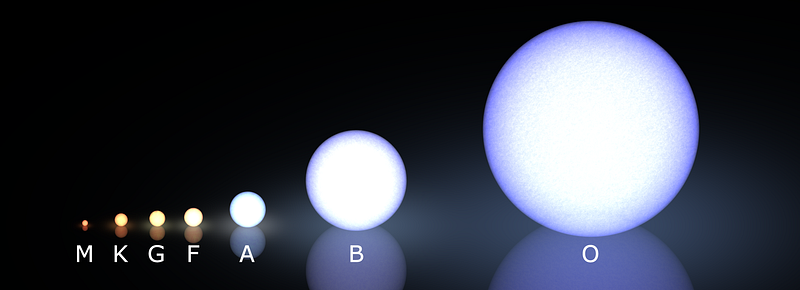
Conversely, there are stars out there with tens or even hundreds the mass of our Sun, yet the most massive among them will live less than 0.01% the amount of time, despite having so much more fuel. For stars, an object with twice the fuel lives only one eighth as long, making our log-on-the-fire problem seem like peanuts!
Thanks for a great question, Pamela, and for the opportunity to explore the science behind a phenomenon that many of us notice, but that defies our intuition. If you’ve got a question you’d like to see featured on Ask Ethan, send yours in here, and we’ll see you next week for more wonders of the Universe right here on Starts With A Bang!
Leave your comments at the Starts With A Bang forum on Scienceblogs!