Gene therapy partially restores function to color receptors in color blind children
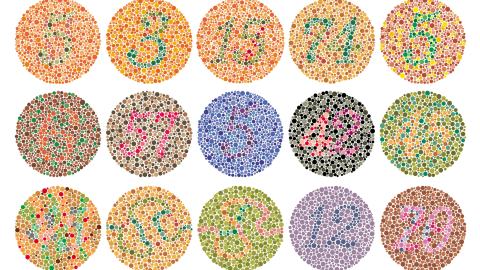
- Total color blindness, also known as achromatopsia, is often caused by mutations that disrupt cone photoreceptor function.
- People with achromatopsia still possess cones, but the mutations prevent the cones from sending signals to the brain.
- Two children with total color blindness underwent gene therapy to correct the mutations, and the treatment partially restored the remaining cones’ function.
It’s hard to imagine life without colors. They are part of our identities, our cultures, and even our emotions. Unfortunately, however, not everyone lives in a colorful world. Fortunately, for those who are color blind, colors may be just around the corner. According to a new study in Brain, gene therapy restored the function of color-detecting receptors in two children, who were born completely color blind, by reawakening dormant neural circuits.
Cause of color blindness
Our eyes detect light with two types of photoreceptors: rods, which are responsible for detecting light intensity, and cones, which are responsible for detecting light color. Both receptors are essential for overall vision and they degrade as we age, which is one of the reasons our vision declines as we get older. Color blindness, however, is caused by defects in cone receptors. Unfortunately, there isn’t a treatment to cure color blindness, but there are special glasses that enhance color vision for individuals with the most common type of red-green color blindness. Still, these glasses are useless for individuals with total color blindness (achromatopsia).
Achromatopsia is rare, occurring in ∼1:30 000 births, and usually is caused by a mutation in one of two genes: CNGA3 (∼30% of European and US cases) and CNGB3 (∼50% of cases). People with total color blindness still have cone photoreceptors; however, these mutations prevent the receptors from sending signals to the brain’s visual cortex. A University College London (UCL) team suspected they could activate the dormant neurocircuitry with gene therapy.
Gene therapy for eye diseases isn’t always effective
Gene therapy targeting inherited eye diseases has proven safe but only sometimes effective. For example, scientists recently used gene therapy to successfully treat Leber’s congenital amaurosis, an inherited eye disease that causes severe early-onset blindness. Additionally, two recent clinical trials that published gene therapy results applied to adults with CNGA3-associated color blindness. These studies tested for improvement of vision, light sensitivity, color thresholds, and visual cortex function after gene therapy. However, the improvements were overall modest.
The UCL team suspected that one reason these effects were modest is that a mature visual system has reduced retinocortical plasticity. In other words, it isn’t very good at incorporating changes. This hypothesis is supported by a 2011 study that reported gene therapy had greater benefits when applied in a young mouse model of color blindness. Therefore, the UCL researchers were interested in learning if gene therapy would work in human children with color blindness.
Gene therapy rescued cone photoreceptor signaling
UCL researchers used MRI brain scans to study four children with total color blindness who received the gene therapy, comparing them to nine untreated patients and 28 volunteers with normal vision. Before the treatment, the scans revealed no cone-mediated signals in the color blind children’s visual cortex. However, six to 14 months after treatment, the imaging showed cones were transmitting signals in two of the children’s visual cortexes. Additionally, the children demonstrated improved visual functions thanks to the newly acquired cone signals. They couldn’t see colors, but their ability to see contrast had improved.
“Our study is the first to directly confirm widespread speculation that gene therapy offered to children and adolescents can successfully activate the dormant cone photoreceptor pathways and evoke visual signals never previously experienced by these patients,” said Tessa Dekker, the lead author of the study. “We are demonstrating the potential of leveraging the plasticity of our brains, which may be particularly able to adapt to treatment effects when people are young.”
One of the patients commented: “Seeing changes to my vision has been very exciting, so I’m keen to see if there are any more changes and where this treatment as a whole might lead in the future.”
The researchers are still analyzing the study’s results, and it remains unclear whether the children will develop the ability to see color. Nevertheless, there is strong evidence that this gene therapy can improve everyday vision for people with total color blindness.