How The Big Bang Failed To Set The Universe Up For The Emergence Of Life

The raw ingredients just weren’t there. Thankfully, their predecessors were.
Here on Earth, our planet practically overflows with life. After more than 4 billion years, life has spread to practically every niche of our planets surface, from the deepest depths of the ocean trenches to the continental shelves to nearly boiling, acidic geothermal springs to the high mountain peaks. Living organisms are literally everywhere, well-adapted to their ecological niches and capable of extracting energy and/or nutrients from their environments to survive and reproduce.
Yet, despite the tremendous differences between an anaerobic single-celled organism and a human being, their similarities are striking. All organisms rely on the same biochemical precursor molecules, which in turn are built out of the same atoms: primarily carbon, nitrogen, oxygen, hydrogen, and phosphorous, with a number of other elements also being essential to life processes. Given that everything in the Universe arose from the same cosmic beginning — the hot Big Bang — you might think that these building blocks were there from the start. But that couldn’t be further from the truth. The Big Bang, spectacular though it was, failed to put the proper ingredients in place for life to arise. Here’s how, for all its successes, the Big Bang failed to set the Universe up for the emergence of life.
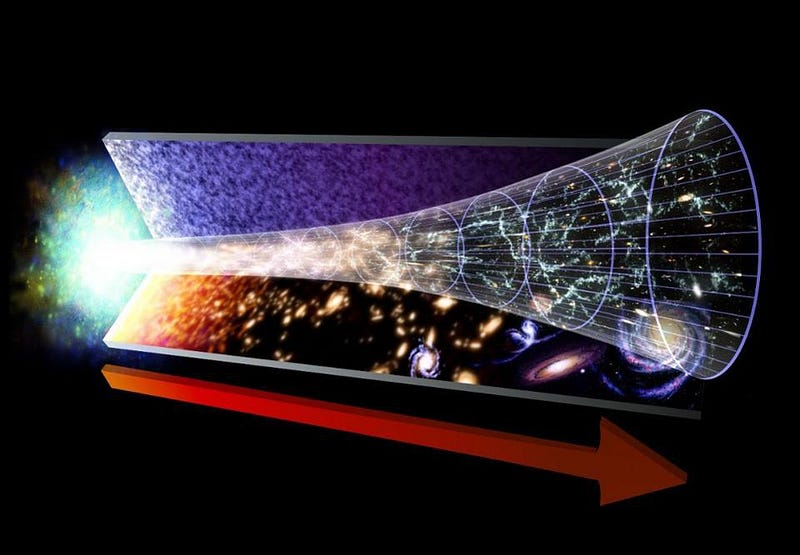
The biggest takeaway from the hot Big Bang is this: the Universe, as it exists today, is cold, expanding, sparse, and clumpy, having emerged from a hotter, more rapidly expanding, denser, and more uniform past.
If this sounds like a wild idea to you, don’t be alarmed; in many ways, it is. The first hint we had that the Big Bang — or something very much like it — might describe our Universe didn’t come from any observable fact, but rather from a theoretical consideration.
If you begin with General Relativity, our best theory of gravity, and you consider a Universe that’s filled with roughly equal amounts of matter everywhere, you’ll discover something fascinating: this Universe is unstable. If you simply started with this matter at rest, the entire Universe would collapse until it created an event horizon and formed a black hole. At this point, the Universe as we know it would end in a singularity. As first realized by Alexander Friedmann way back in 1922, a Universe filled with equal amounts of “stuff” everywhere could not be both stable and static; it must either be expanding or contracting.
Observationally, the 1920s became a revolutionary decade for our understanding of the Universe. Newer, larger, more powerful telescopes enabled us to measure, for the first time, the properties of individual stars in galaxies other than the Milky Way, revealing their distances. Combined with the fact that the light we observed from them was not only systematically shifted towards longer, redder wavelengths, but that the farther a galaxy was from us, the greater the redshift was, this helped seal the deal: the Universe was expanding.
If the Universe is expanding today, and the light traveling through it was getting stretched to longer, redder wavelengths, then that teaches us that our Universe will continue to get:
- larger in volume,
- less dense in terms of the matter-and-energy per unit volume,
- clumpier as gravitation continues to draw nearby masses towards one another,
- and colder, as the light traveling through it becomes continuously lower in temperature.
If we know what the Universe is made out of, we can even figure out how that expansion rate will evolve into the far future.
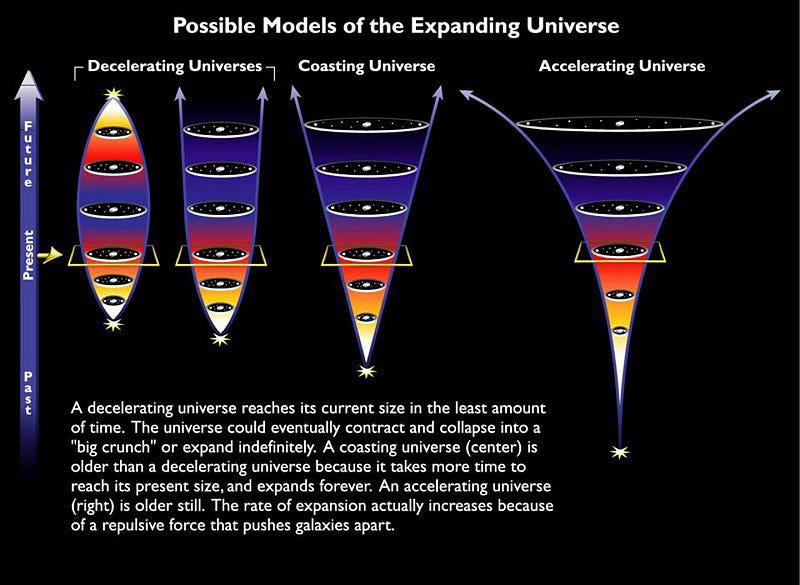
But something remarkable comes along for the ride: if we can figure out what the Universe is made out of and how it’s expanding today, we can not only extrapolate the far future of the Universe, but also the distant past as well. The same equations — the Friedmann equations — that tell us how the Universe will evolve into the future also tell us what the Universe must have been like in the past; remember that in General Relativity, spacetime tells matter and energy how to move, while matter and energy tell spacetime how to curve and evolve.
If you know where all the matter and energy is and what it’s doing at any moment in time, you can determine how the Universe expanded and what its properties were at any point in either the past or the future. If we step backwards in time, then, instead of forwards, we’ll find that the young Universe should be:
- less clumpy and more uniform,
- smaller in volume and greater in matter-and-energy density,
- and hotter, as the radiation within it has had less time to be shifted to lower energies.
This last part extends not only to the light and radiation created by stars, but to any radiation that’s been present throughout all of our cosmic history, including even the very beginning.
If you imagine starting off the Universe in a very hot, dense, and uniform state, but one that’s expanding very rapidly, the laws of physics themselves will paint a remarkable picture of what’s to come.
- In the initial stages, every quantum of energy that exists will be so hot that it will be traveling at speeds indistinguishable from the speed of light, smashing into other quanta countless times per second due to the overwhelming densities.
- When a collision occurs, there’s a substantial chance that any particle-antiparticle pair that can get created — restricted only by the quantum mechanical conservation laws that govern the Universe and the amount of energy available for particle creation from Einstein’s famous E = mc2 relation — will come into existence.
- Similarly, whenever a particle-antiparticle pair happen to collide, there’s a substantial chance that they’ll annihilate back into photons.
So long as you have an initially hot, dense, expanding Universe filled with interacting quanta of energy, those quanta will populate the Universe with all the various types of particles and antiparticles that are permitted to exist.
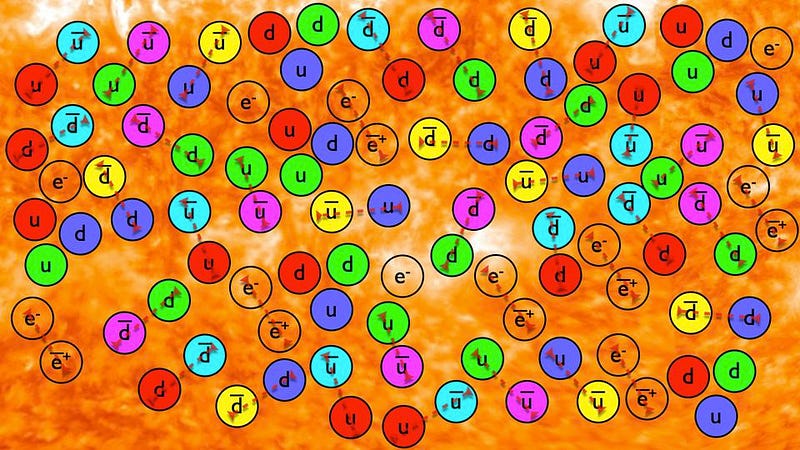
But what happens next? As the Universe expands, everything cools: massive particles lose kinetic energy while massless particles get redshifted to longer wavelengths. Early on, at very high energies, everything was in equilibrium: particles and antiparticles got created at the same rate they got annihilated. But as the Universe cools, the “forward” reaction rates, where you create new particles-and-antiparticles based on collisions, begin to occur less rapidly than the “backwards” reaction rates, where particles-and-antiparticles annihilate away back into massless particles, such as photons.
At very high energies, all of the known particles and antiparticles of the Standard Model are easy to create in large quantities. As the Universe cools, however, the more massive particles and antiparticles become more difficult to create, and they eventually annihilate away until there’s a negligible amount left. This winds up leading to a Universe filled with radiation, with just a tiny bit of leftover matter: protons, neutrons, and electrons, which somehow came to exist slightly more abundantly — about 1 extra matter particle per 1.4 billion photons — than antimatter. (How, exactly, that occurred is still an open area of research, and is known as the baryogenesis problem.)
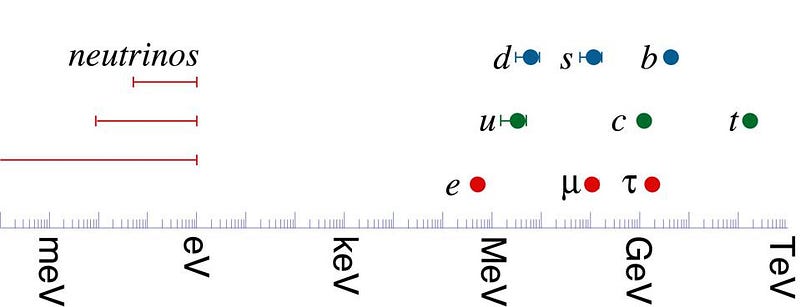
About 1 second after the Big Bang, the Universe is still very hot, with temperatures in the tens of billions of degrees: about ~1000 times hotter than in the center of our Sun. The Universe still has a little bit of antimatter left, because it’s still hot enough for electron-positron pairs to be created as quickly as they’re destroyed, and because neutrinos and antineutrinos are as equally copious as one another, and almost as copious as photons. The Universe is hot and dense enough for the remnant protons and neutrons to begin the process of nuclear fusion, building their way up the periodic table to create the heavy elements.
If the Universe could do precisely this, then as soon as the Universe becomes cool enough to form neutral atoms and enough time passes so that the gravitational imperfections can attract enough matter to form stars and star systems, we’d have chances for life. The atoms necessary for life — the raw ingredients — can bind together into all sorts of molecular configurations all on their own, through natural, abiotic processes, just like we find today all throughout interstellar space.
If we could begin building elements in these early stages of the hot Big Bang, the high temperatures and densities could permit not just fusion of hydrogen into helium, but helium into carbon, and so on into nitrogen, oxygen, and many of the heavier elements found all throughout the modern cosmos.
But that’s a big “if,” and one that doesn’t turn out to be true.
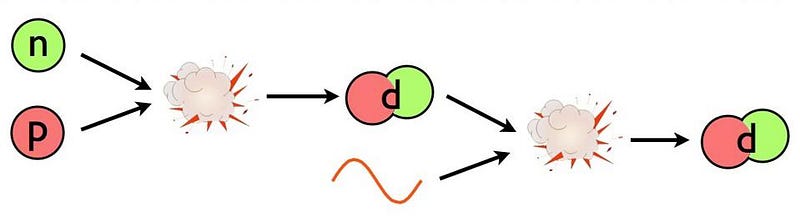
This is the problem: deuterium. The Universe is full of protons and neutrons, and it’s hot and dense. Whenever a proton and neutron find one another, they’ll fuse into a deuteron, which is a heavy isotope of hydrogen, and is also more stable than a free proton and neutron separately; each time you form a deuteron from a proton and neutron, you liberate 2.2 million electron-volts of energy. (You can also form deuterium from nuclear reactions involving two protons, but the reaction rate is much lower than from a proton and a neutron.)
So why, then, can’t you add protons or neutrons to each deuteron, building your way up to heavier isotopes and elements?
The same hot, dense conditions lead to a “backwards” reaction that swamps the “forward” creation of deuterium by fusing protons with neutrons: the fact that enough photons, which outnumber protons and neutrons by more than a billion-to-one, have more than 2.2 million electron-volts of energy themselves. When they collide with a deuteron, which occurs far more frequently than a deuteron colliding with anything else made out of protons-and-neutrons, they immediately blast it apart.
The inability of the cosmos to maintain deuterium in the early Universe for long enough periods to build up to heavier elements is the primary reason that the Big Bang can’t create the ingredients for life on its own.
So, what can the Universe do? It’s compelled to wait until it’s expanded and cooled enough so that deuterium isn’t immediately blasted apart. But in the meanwhile, a whole slew of other things happen while we wait for the Universe to cool sufficiently. They include:
- neutrinos and antineutrinos stop efficiently participating in interactions with other particles, also known as the freeze-out of the weak interactions,
- electrons and positrons, like other species of matter and antimatter, annihilate away, leaving only the excess electrons,
- and the free neutrons, being unable to bind themselves up in heavier nuclei, begin to decay away into protons, electrons, and anti-electron neutrinos.
Finally, after a little more than about ~200 seconds, we can finally form deuterium without immediately blasting it apart. But at this point, it’s too late. The Universe has cooled but become much less dense: only about one-billionth the density found in the central core of our Sun. The deuterons can fuse with other protons, neutrons, and deuterons to build up copious amounts of helium, but that’s where the chain reaction ends.
With less energy per particle, with strong repulsive forces between the helium nuclei, and with every combination of:
- helium-4 and a proton,
- helium-4 and a neutron,
- and helium-4 and helium-4,
being unstable, that’s pretty much the end of the line. The Universe, in the immediate aftermath of the Big Bang, is made of 99.99999%+ hydrogen and helium, exclusively.
Even though we’re talking about cosmic scales, it’s actually the laws that govern subatomic particles — nuclear and particle physics — that prevents the Universe from forming the heavy elements required for life in the early stages of the Big Bang. If the rules were a little bit different, like deuterium was more stable, there were much greater numbers of protons and neutrons, or there were fewer photons at high energies, nuclear fusion could have built up large quantities of heavy elements in the first few seconds of the Universe.
But the easily-destroyed nature of deuterium, combined with the enormous numbers of photons present in the early Universe, kills our dreams of having the necessary raw ingredients right at the beginning. Instead, it’s just hydrogen and helium, and we’ll have to wait hundreds of millions of years for stars to form before we build up any substantial quantities of anything heavier. The Big Bang was a great start to our Universe, but couldn’t set us up for life all on its own. For that, we needed generations of stars to live, die, and enrich the interstellar medium with the heavier elements that all biochemical processes require. When it comes to your existence, the Big Bang absolutely isn’t enough to give rise to you. For that to occur, you can literally thank your lucky stars: the ones that lived, died, and created the essential elements still inside you today.
Starts With A Bang is written by Ethan Siegel, Ph.D., author of Beyond The Galaxy, and Treknology: The Science of Star Trek from Tricorders to Warp Drive.