Ask Ethan: How Fast Could Life Have Arisen In The Universe?

It took the Universe 9.2 billion years to create the Earth, and another 4 billion for complex life. Could we have gotten there faster?
The story of how the Universe came to be the way it is today, from the Big Bang to the vast void of space littered with clusters, galaxies, stars, planets, and life, is the one story we all have in common. From our perspective here on Earth, it took about 2/3rds of our shared cosmic history before the Sun and Earth were even created. Yet life appeared on our world as far back as we’re able to measure: perhaps as much as 4.4 billion years ago. It makes one wonder if life in the Universe predated our planet, and, for that matter, how far back life could possibly go? That’s what Matt Wedel wants to know, as he asks:
How soon after the Big Bang would there have been enough heavy elements to form planets and possibly life?
Even restricting ourselves to the type of life that we would recognize as being “like us,” the answer to this question goes back farther than you might ever imagine.
We can’t go back to the very beginning of the Universe, of course. In the aftermath of the Big Bang, there were not only no stars or galaxies to start with, but there weren’t even atoms. Everything takes time to form, and the Universe, born containing a sea of matter, antimatter, and radiation, began as a mostly uniform place. The densest regions were only a tiny fraction of a percent — perhaps 0.003% — denser than average. This means it will take a tremendous amount of gravitational collapse to create something like a planet, which is around 1030 times denser than the mean density of the Universe. And yet, the Universe is free to take exactly as much time as it needs to make it all happen.

After the first second-or-so, the antimatter has all annihilated away with most of the matter, leaving just a tiny bit of leftover protons, neutrons, and electrons amidst a sea of neutrinos and photons. After 3–4 minutes, the protons and neutrons have formed stable atomic nuclei, but it’s almost all isotopes of hydrogen and helium. And it’s only when the Universe sufficiently cools below a particular threshold, which takes approximately 380,000 years, that we can bind electrons to these nuclei, forming neutral atoms for the first time. Even with these fundamental ingredients in place, life — and even rocky planets — are not yet possible. Hydrogen and helium atoms alone simply will not do.
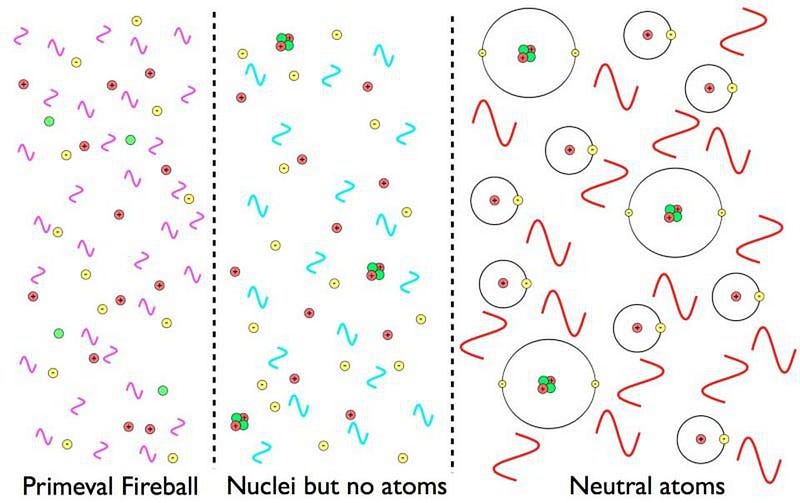
But gravitational collapse is a real thing, and given enough time, it will change the Universe. Even though it happens slowly at first, it’s relentless and it builds upon itself. The denser a region of space gets, the better it becomes at attracting more and more matter to it. The regions that begin with the greatest overdensities grow the fastest, with simulations indicating that the very first stars of all should form somewhere around 50–100 million years after the Big Bang. These stars should be made of hydrogen and helium exclusively, and ought to be capable of growing to very large masses: hundreds or perhaps even a thousand times the mass of our Sun. And when a star this massive forms, it’s a matter of only perhaps one or two million years before those stars die.
But what goes on when those stars die is tremendous, because of how those stars lived. All stars fuse hydrogen into helium in their cores, but the most massive ones not only fuse helium into carbon, but then carbon into oxygen, oxygen into neon/magnesium/silicon/sulfur, and then on and on up the periodic table until you get to iron, nickel and cobalt. After that, there’s nowhere else to go, and the core collapses, triggering a supernova explosion. These explosions recycle huge amounts of now-heavy elements into the Universe, triggering new generations of stars and enriching the interstellar medium. All of a sudden, heavy elements, including the ingredients we need for rocky planets and organic molecules, now fill these proto-galaxies.

The more stars live, burn, and die, the more enriched the next generation of stars will be. Many supernovae create neutron stars, and it’s neutron star-neutron star mergers that create the largest amounts of the heaviest elements in the periodic table. Greater fractions of heavy elements mean more rocky planets of greater density, greater amounts of the elements essential to life-as-we-know it, and greater probabilities for complex organic molecules to take place. We don’t need the average place in the Universe to look like our Solar System; we simply need for a few generations of stars to live and die in the densest regions of space in order to produce the conditions for rocky planets and organic molecules.
By time the Universe is just one billion years old, the most distant objects we can measure the heavy element abundance for have huge amounts of carbon: as much as our own Solar System contains. The other heavy elements become more abundant even more quickly; carbon perhaps takes more time to reach a high abundance because it’s primarily produced in stars that don’t go supernova, rather than the ultra-massive ones which do. Rocky planets don’t need carbon; other heavy elements will do just fine. (And many supernovae will create phosphorous; don’t worry about recent reports falsely exaggerating its absence.) It’s quite likely that only a few hundred million years after the first stars turned on — by time the Universe is 300 to 500 million years old — we had rocky planets forming around the most enriched stars at the time.

If it weren’t for the necessity of carbon for life, there would likely be regions of space that could have begun life processes at that time as well. But we need carbon for life like ours, and that means we have to wait a little longer for a good possibility to have life. Although carbon atoms will be present, a large enough abundance will probably require waiting between 1–1.5 billion years: until the Universe is about 10% of its present age, rather than the 3–4% it requires for rocky planets. It’s interesting to think that the Universe formed planets and had all the ingredients in the right abundances to create life except for carbon, and that it takes the life-and-death of the most massive Sun-like stars to give us enough of the most important life-giving ingredient of all.
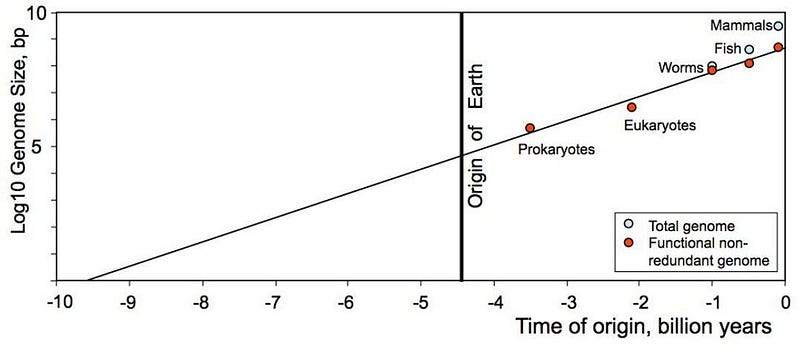
It’s an interesting exercise that if you extrapolate back the most advanced forms of life we find on Earth at various epochs in our planet’s history, you’ll find that the genomes have a complexity that increases with a particular trend. If you go all the way back to single base pairs, however, you get a figure that’s closer to 9–10 billion years ago than 12–13 billion years ago. Is this an indication that the life we have on Earth began well before Earth did? And furthermore, is it an indication that life could have began billions of years earlier, but where we are it took an extra few billion years to get started?
At this point, we don’t know. But at the same time, we also don’t know where that line is between life and non-life. We also don’t know if terrestrial life got its start here, on an earlier planet, or if it began in the depths of interstellar space, without a planet at all.

What’s incredibly interesting, though, is that the raw elemental ingredients necessary for life began existing back shortly after the first stars formed, and the most important ingredient — carbon, the fourth most common element in the Universe — is actually the very last ingredient to come about in the abundance we need. Rocky planets, at least in some locations, come about much earlier than life can: just half a billion years after the Big Bang, or perhaps even sooner. Once we have carbon, however, 1-to-1.5 billion years after the Big Bang, all the steps we need to take to produce organic molecules and the first steps towards life are inevitable. Whatever life processes took place to lead to humanity’s existence, as best as we understand them, could have begun when the Universe was just one-tenth the age it is now.
Send in your Ask Ethan questions to startswithabang at gmail dot com!
Ethan Siegel is the author of Beyond the Galaxy and Treknology. You can pre-order his third book, currently in development: the Encyclopaedia Cosmologica.