Ask Ethan #34: Using up the Universe’s fuel
Hydrogen was the first element ever created, but there’s less of it now than there ever was.
“If the human condition were the periodic table, maybe love would be hydrogen at No. 1.” –David Mitchell
Some weeks, the questions that we pick for our weekly Ask Ethan column are about phenomena here on Earth, ranging from human concerns like education to engineering to the physical state of the planet itself. But other weeks, we go far into the Universe, and consider the stars, galaxies, or the entire Universe as a whole, from the known all the way to the unknowable. You’ve all continued to send in your questions and suggestions, and this week’s chosen entry comes from Franklin Johnston, who asks us to think about how some of the smallest bits of the Universe have evolved on the largest (and longest) scales:
What’s our current understanding as to how much hydrogen was initially created after the Big Bang, and what’s happened to it since? I’d like to know how much is currently in stars, how much has been converted to heavier elements, how much in planets, moons, and comets, how much in interstellar space, how much in intergalactic space, and any place else I may have overlooked.
There’s only one way to start, and that’s to begin at the very beginning of our observable Universe as we know it: at the Big Bang itself!
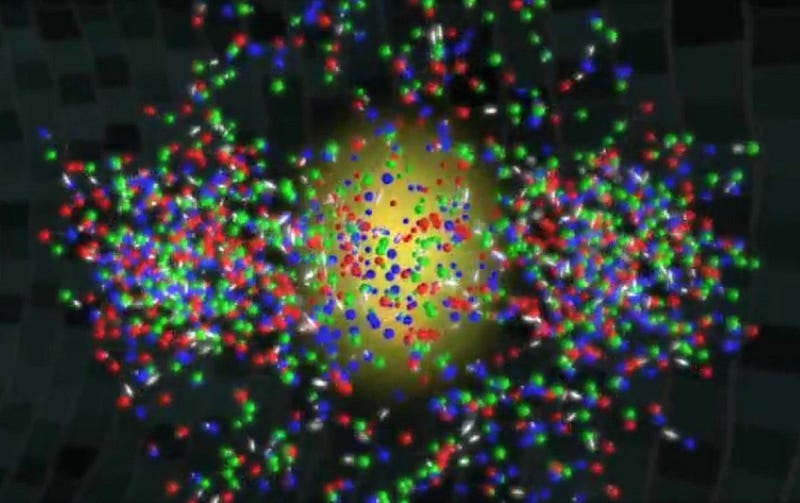
When cosmic inflation ended, and all the energy that had been locked up as energy intrinsic to space itself was transformed into matter, antimatter and radiation, what we traditionally think of as our observable Universe began. Full of a hot, dense soup of ultrarelativistic particles, it began to cool as it expanded, and the expansion rate slowed tremendously over time. Matter won out over antimatter (and the remainder annihilated away), and quarks and gluons came together to form free protons and neutrons, all amidst a sea of radiation far more numerous than the protons and neutrons that would come to make up the bulk of what we know as “normal matter” in our everyday parlance.
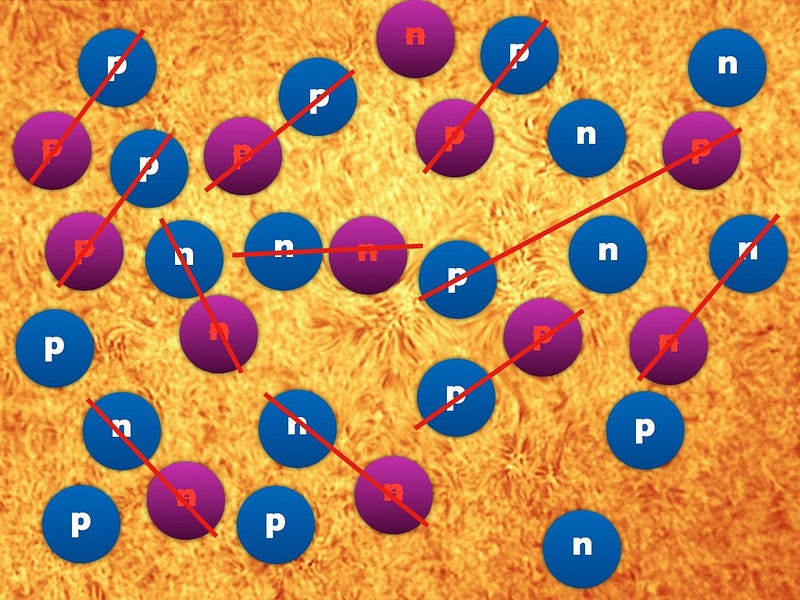
By time a single second had passed since that hot Big Bang began, the part of the Universe that’s observable to us today contained around 10^90 radiation particles, with about 10^80 protons and neutrons (split approximately 50/50) left over. The majority of neutrons wound up either converting into protons via neutrino capture or via radioactive decay, and by time the Universe was a little over three minutes old, the remaining neutrons would fuse together with the protons to form helium.
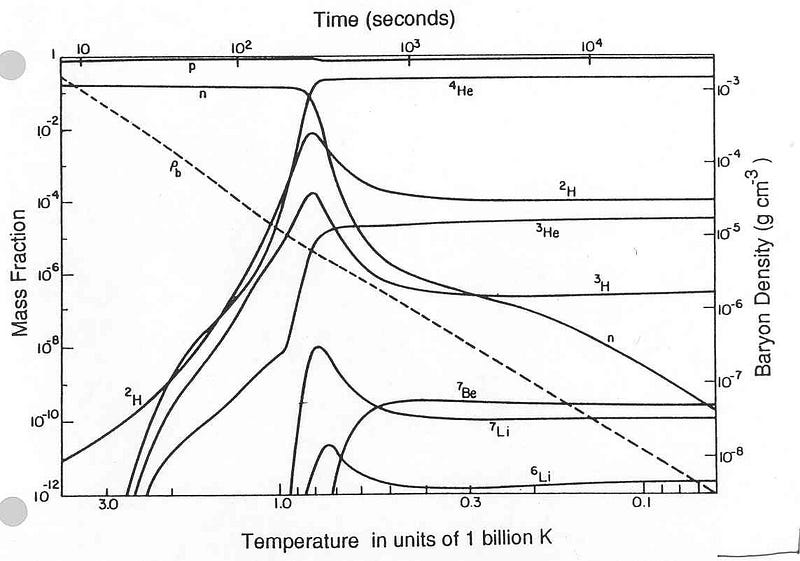
By the time the Universe was four minutes old, 92% of all atomic nuclei, by number, were hydrogen atoms, with the remaining 8% as helium. (If you were to classify these atoms by mass instead, considering that helium is typically four times as massive as hydrogen, the split is more like 75%/25%.)
Over even more time, the Universe continued to cool, forming neutral atoms after a few hundred thousand years, and then — over millions of years — those neutral atoms cooled and contracted to form giant molecular gas clouds. Although the electromagnetic and gravitational forces have interesting effects during this time, it takes a nuclear reaction to change the type of atom you have. So nothing really changes during this time as far as hydrogen is concerned. That is, of course, until the first stars form.

Whenever you make a true star, its defining feature is that in its core, it begins fusing lighter nuclei into heavier ones. This process of nuclear fusion only occurs under the tremendous temperatures, pressures and at the high densities when at least tens of thousands of Earth masses worth of hydrogen get together in a single bound structure. When the temperature of the core exceeds about four million Kelvin, fusion can begin, and the first stage in fusion is single protons — the nuclei that define hydrogen — working their way up the nuclear chain to eventually form helium. There are other reactions that can take place later on, but today’s focus is on hydrogen.
How long does it take to eat up this hydrogen? The biggest determining factor, believe it or not, is actually pretty straightforward: the mass of the star when it first forms.

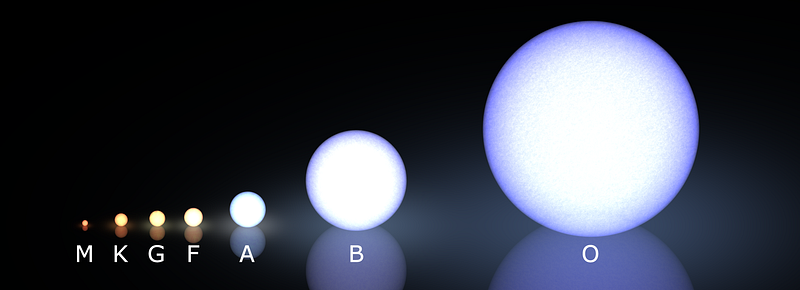
For the highest-mass stars, the ones that are hundreds of times the mass of our Sun (such as the brightest, bluest ones shown above), their cores burn through their hydrogen incredibly fast, using it up in a matter of just a few million years at most. These O-class stars are very rare, making up less than 0.1% of all stars, but they are the brightest and most luminous stars in the entire Universe, and also the fastest places for the Universe to use up its hydrogen.
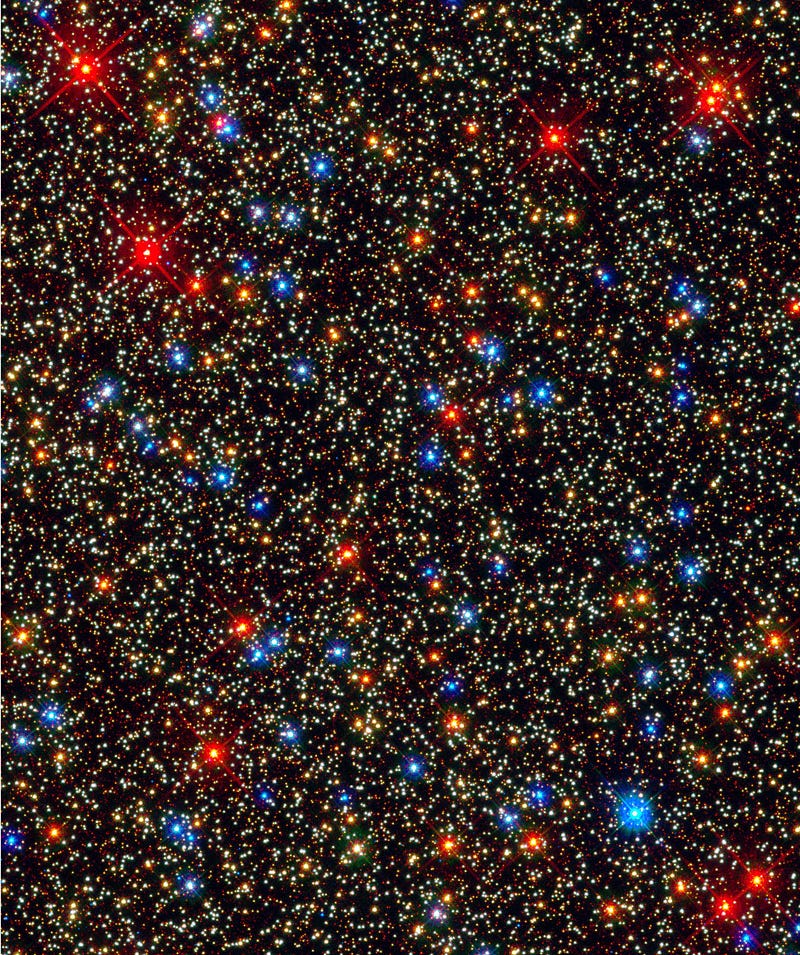
On the other hand, the lowest mass stars — main sequence M-class stars far too dim to appear even in the Hubble image above — might live for tens or even hundreds of trillions of years (more than 1,000 times the present age of the Universe) before they burn through all of their hydrogen. It might not seem all that important on the surface, but don’t forget that M-class stars are by far the most common stellar type in the Universe; three out of every four stars that are alive today are M-class stars!
You might think that, given all the generations of stars that have lived and died over the past 13.82 billion years, and given the vast abundance of elements heavier than hydrogen here on Earth and throughout the Solar System, there’d be a lot less hydrogen in the Universe today.
Yet that’s simply not the case.
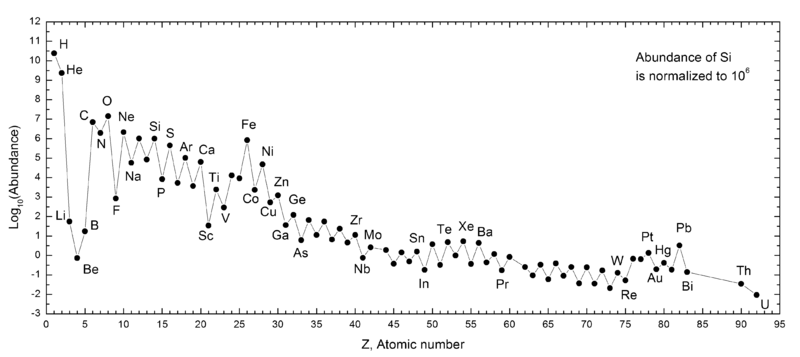
Our Sun is significantly enriched, having formed when the Universe was more than 9 billion years old in the plane of a spiral galaxy, one of the most enriched places in the Universe. Yet, when our Sun formed, it was still made out of — by mass — 71% hydrogen, 27% helium, and about 2% “other” stuff. If we convert that into “number of atoms” and treat the Sun as typical of the Universe, that means, over the first 9.3 billion years of the Universe, the fraction of hydrogen has gone down from 92% to 91.1%.
That’s it. So how is that change so tiny?

When a molecular cloud collapses to form stars, only about 5-to-10% of the mass of the initial cloud will wind up in stars. The vast majority of the rest gets blown back out into the interstellar medium by the ultraviolet radiation emitted by the hot stars that form earliest.

And then, on top of that, all of the stars heavier than M-class stars only burn about 10% of their total fuel before expanding into a red giant. For the lowest mass (M-class) stars, the “burn” is slow enough that the entire star has time to convect, moving the burned fuel from the core into the outer layers, and to move unburned hydrogen into the core; a star like Proxima Centauri will eventually turn 100% of its hydrogen into helium, a process that will take a few trillion years.
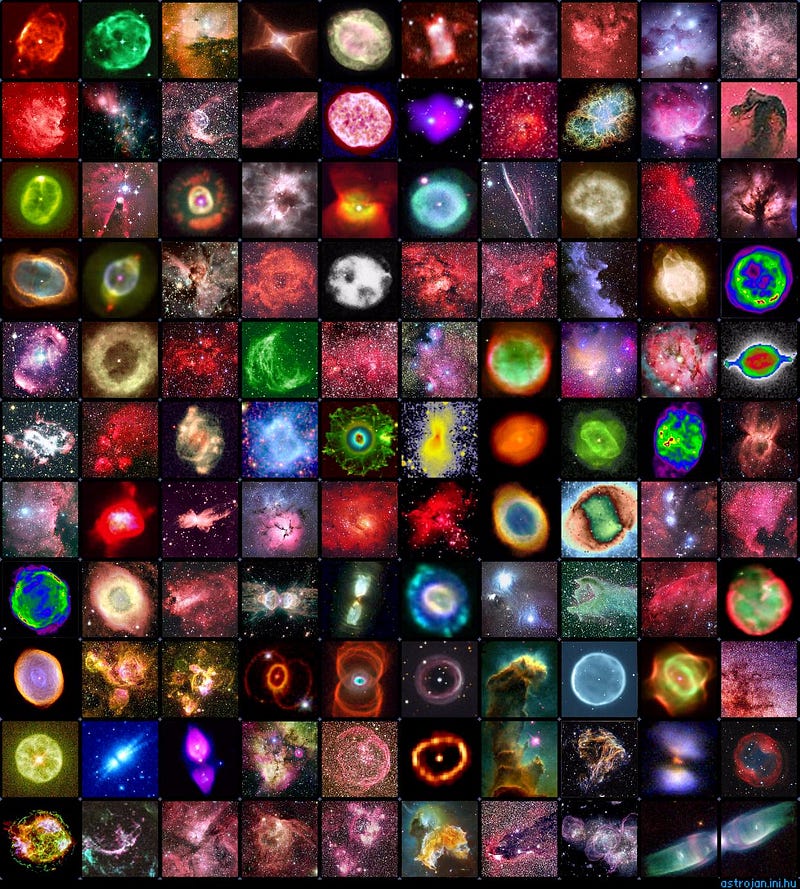
But every star that belongs to a heavier class will burn just 10% of its hydrogen fuel, die in either a supernova or planetary nebula, and return the vast majority of its unburned fuel back to the interstellar medium.
And yet amidst all this, galaxies merge, and go through intense periods of star formation when that happens, known as starbursts.

Yet the more violent these starbursts are, the more hydrogen actually gets expelled from the galaxy entirely, thrown into the intergalactic medium! At this point in time, about 50% of the Universe’s hydrogen is not bound to any galaxy at all, but is rather occupying the space between galaxies, and will very likely never form stars again. On top of all of that, the overall star formation rate has dropped tremendously over the history of the Universe; from its maximum, the rate the Universe forms new stars is just 3% of what it once was.

And yet, galaxies remain as bound structures, and will continue to have very large amounts of hydrogen far into the future. Even though it very likely won’t create new stars by the same mechanism that dominates today, we expect there to be new stars for many trillions of years (hundreds or thousands of times the present age of the Universe), and possibly for significantly longer.
The Universe will go dark, but it won’t be because it ran out of hydrogen. Rather, it will be because the hydrogen that is left is unable to bind together in a large enough molecular cloud to form new stars. It’s only an estimate, but I doubt that — by number of atoms — the amount of hydrogen in the Universe will ever drop below 80%. In other words, we’re going to form plenty of helium and a large number of heavier elements, but at all moment in time, even if we ran the theoretical clock to infinity, the Universe will always be mostly hydrogen. (Which shouldn’t be too surprising; by number-of-atoms, you are mostly hydrogen!)
By mass, we may wind up with less than 50% of the Universe as hydrogen, particularly due to large galaxies and clusters of galaxies. The fact of the matter is, when the Universe is millions of times its present age, we fully expect that new stars will still be forming, but by a very different mechanism by collapsing molecular clouds millions of times the mass of the Sun.

Will that process run to near-completion? We don’t have the theoretical or computational power to know, and the Universe hasn’t been around for long enough for us for observations to give us any useful information.
But to the best of our knowledge, hydrogen started off as the most abundant element in the Universe, and it will remain that way for as long as there’s a Universe to exist in. Thanks for a fun question, Franklin, and if you’d like the chance to be the subject of the next Ask Ethan, send in your questions and suggestions here!
Leave your comments at the Starts With A Bang forum on Scienceblogs!