FDA Approves New Device, Allowing the Blind to See

What’s the Latest Development?
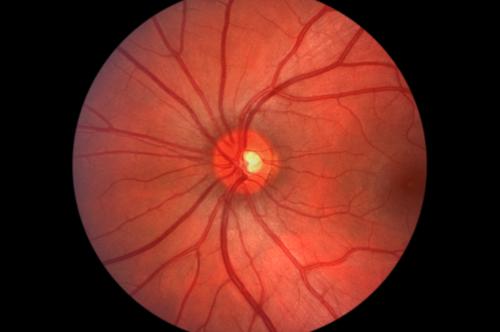
The Food and Drug Administration has approved the first-ever device that works like an artificial retina to help blind people see shapes, shadings and even distinct colors. “The artificial retina is a sheet of electrodes implanted in the eye. The patient is also given glasses with an attached camera and a portable video processor. This system, called Argus II, allows visual signals to bypass the damaged portion of the retina and be transmitted to the brain.” While the device does not allow the blind to see in a conventional sense, contrast between light and dark allows them to identify the outlines and boundaries of objects.
What’s the Big Idea?
In everyday life, the device will allow the blind to recognize street crosswalks, the presence of cars and large numbers or letters indicating an address. The new vision technology was developed over 20 years by Dr. Mark S. Humayun, an ophthalmologist and biomedical engineer at the University of Southern California. “Dr. Humayun said he envisioned applying the technology to other conditions than blindness, implanting electrodes in other parts of the body to address bladder control problems, perhaps, or spinal paralysis. ‘We don’t think of the human body as an electrical grid, but it runs off electrical impulses,’ he said.”
Photo credit: Shutterstock.com