Yes, It’s Hot; But No, Your Gas Tank Won’t Explode If You Fill It Up

Fill up your gas tank, no matter how hot it is, with confidence and safety from anywhere on Earth.
For most of the northern hemisphere, summer is in full swing. Along with the customary scorching temperatures and wildly uncomfortable conditions for people, there are heat advisories put out by organizations like the National Weather Service to keep people safe.
But it isn’t just humans and other animals that are at risk from the heat. As many locations dry out, the fire risk increases tremendously. Burn bans are put into place in dozens of counties. Enclosed spaces are a danger, too; they can get extremely hot very quickly, such as inside of a closed car. While many warnings exist about these dangers, a new, troubling warning has surfaced: claims that a full gas tank can spontaneously explode on a hot day. The warnings are going viral in many parts of the world, but is this a legitimate concern? Let’s look to the science to find out.

First off, there’s absolutely a physical element of truth here. If you take any closed container of a fixed volume, and pump enough gas under enough pressure into it, it will eventually explode. This is the same principle behind an overinflated balloon popping: when the interior, outward-pushing pressure is too great for the container’s walls to withstand, a rupture will occur, and the high-pressure gas trapped inside will explode.
For a spectacular example of this, simply put some dry ice in an empty soda bottle, screw the cap on tightly, and stand back. As the dry ice sublimates, the bottle will fill (and then overfill) with CO2 gas. When a critical pressure is reached, the soda bottle will give out, and the makeshift pressure bomb will detonate.
With gasoline in a filled automobile fuel tank, the danger is much more palpable. Instead of mere shrapnel, you’d have somewhere around fifteen gallons of highly flammable, explosive material all in one container. If the pressure and temperature increased to such a degree that it ignited, it could release all of that stored energy at once. A gallon of gasoline, quite surprisingly to many, contains a whopping 33.4 kilowatt-hours of energy inside of it
This means, if you were to detonate it and burn all of that energy in a single moment, 15 gallons of gas would create an explosion that’s the equivalent of 860 pounds (390 kg) of TNT. That’s a lot of explosive energy stored in a typical, full gas tank!

But in order to get an explosion of any type, you’d need one of two things to occur:
- You could build up enough pressure to make a pressure bomb, similar to the sublimating dry ice in a soda bottle. This could blow a hole in the gas tank and cause the release of fumes.
- You could heat the gasoline up to a high enough temperature that it could ignite spontaneously: without even a spark.
Either one of these could result in catastrophe. Fortunately, neither one of them can occur at even the hottest temperatures ever achieved on Earth.

It’s very important to prevent the build-up of unwanted pressure; as any Simpsons fan will recall, venting prevents explosion. Up until the 1960s, automobiles were designed with vented gas caps. This was the simplest possible solution to prevent pressure build-up: the gas cap had a hole in it. Without a closed system, pressure build-up was impossible, as air could get out-or-in to stabilize the pressure.
However, since this also allowed fuel to leak out or splash around, they began to be phased out in favor of evaporative emission control (EVAP) systems. Instead of equilibrating with the outside air, there would be a gas line to a holding mechanism that would keep the pressure stable. It would hold fuel when pressures were high, and return them to the main tank when pressures returned to normal.
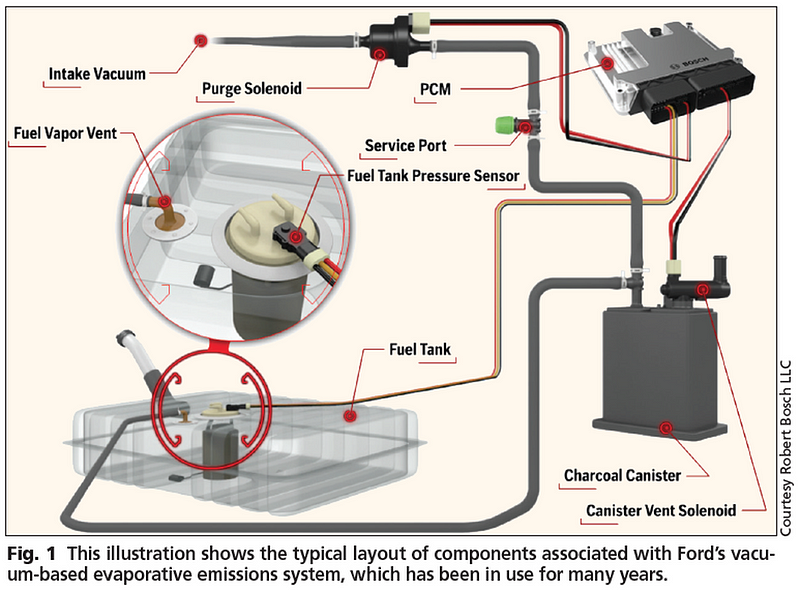
The EPA instituted a rule in 1971 that all vehicles with an internal combustion engine must have an EVAP system. Even if you were to install a non-vented gas cap on a car without an EVAP system, or the EVAP system were to malfunction, there still isn’t the potential for the pressures to build to great enough quantities to cause a rupture. The act of simply opening your gas cap periodically to refuel ensures that. At the conditions reached here on Earth, a gas tank will be impervious to such an explosion.
What, then, of the other option? Of the possibility that the fuel inside the gas tank could spontaneously ignite?

This is a legitimate concern, since temperatures in closed spaced do rise enormously. But you must be careful to distinguish between two important temperatures:
- flash point, which is the lowest temperature that vapors will ignite at given an ignition source (like a spark), and the
- autoignition temperature, which is the temperature that vapors will spontaneously ignite at without an ignition source.
For fuel in your automobile, you want a low flash point (so your car starts even when it’s cold), but a high autoignition temperature, so that you don’t get spontaneous ignition under any conditions. You couldn’t ask for a better fuel, for those purposes, than conventional gasoline.
Its flash point is remarkably low: −45 °F (−43 °C), meaning that even under very cold, winter conditions, your vehicle should still start. But the autoignition temperature is extremely high: 536 °F (280 °C): higher than both diesel engine and jet fuel. The maximum temperature reached inside a running engine on an extremely hot day is around 270 °F (130 °C), which is hundreds of degrees too low to cause autoignition.
The only danger you have from a full gas tank on a hot day is the same danger you have every day: if you have a leaky gas line and a spark, the potential for disaster is catastrophically large.

If gasoline was truly a danger of exploding from the build-up of extremely high pressures, leaving it half-full wouldn’t be much of a countermeasure. Just as a single cube of dry ice the size of your knuckle can create a pressure bomb out of a soda bottle, a volatile liquid that could change into gas could cause a similar effect. Thankfully, gasoline reaches equilibrium at very low pressures relative to the stability of a gas tank, and both vented caps and EVAP systems are more than sufficient to maintain a constant, low pressure. The danger of spontaneous ignition is nil at temperatures achieved on Earth; if we were on Venus or Mercury, the story would be different. (But then again, so would our cars.) There are many dangers on a hot day that we should be concerned about and take precautions against. When it comes to fuel, a full gas tank poses no danger, but running out of gas in the heat can be deadly. Fill up with confidence, carry water, and be safe out there.
Ethan Siegel is the author of Beyond the Galaxy and Treknology. You can pre-order his third book, currently in development: the Encyclopaedia Cosmologica.





