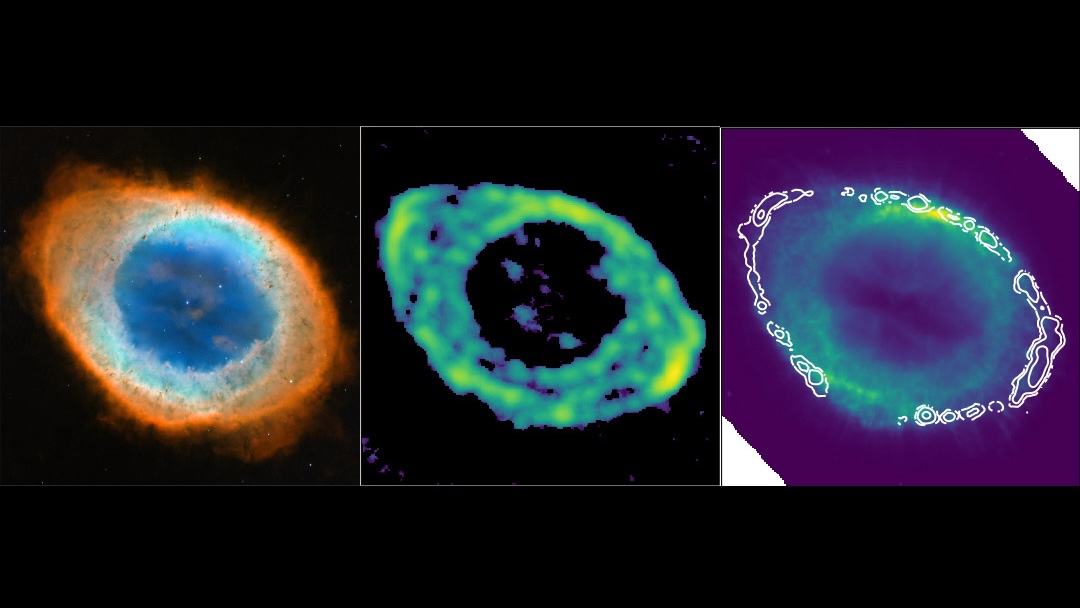
Was Life On Earth Brought Here From An Alien System?

And could Earth-based life provide the seeds for biology elsewhere?
Today, on Earth, there’s an enormous variety and diversity of life on our planet. Every single surviving lifeform appears, in some fundamental way, to be related to every other lifeform; life appears to have a universal common ancestor. As we go farther and farther back in time — from the fossil record, for example — we can see that life was:
- less complex,
- less differentiated,
- had smaller numbers of unique sequences in its genetic code,
- and, if we go back before a certain critical point, lacked many of the developments that we now perceive as critical in leading to human beings.
Before a certain point, mammals didn’t exist. Before that, life only existed in the water, not on land. Prior to that, sex hadn’t evolved; prior to that, all organisms were merely single-celled. And yet, as far back as we can trace it on Earth, we have never yet come to an epoch where we can say with any degree of certainty that life did not exist. It raises a tremendous possibility: that the life that began on Earth originated elsewhere in the Universe, before even the formation of Earth. Not only is that possible, but it’s possible that life, as it evolved on Earth, is now providing the seeds life elsewhere in the galaxy and Universe.
This idea, known as panspermia, was once ridiculed as pseudoscience, but is now firmly back in the scientific mainstream. Here’s the science of why we have to keep this fascinating, speculative, but compelling possibility in mind.
Here on Earth, the surface, the oceans, the atmosphere, and even the submerged deep and the subterranean underground all teem with life. In addition to single-celled life forms, there are macroscopic fungi, plants, and animals pervading the planet’s biosphere. As we go farther back in time, we can learn that life has gotten more complex over time, but that we have yet to encounter an epoch on Earth where our planet was devoid of life.
We typically think of the evidence for past life on Earth as coming from fossils, which get created when sediment — typically in aqueous, underwater environments — gets deposited atop living organisms. As the sediment solidifies into sedimentary rock, the organisms decompose, leaving their fossilized remains imprinted into the rock. For as far back as we have sedimentary rock in the geological history of Earth, we find that they contain fossils. While many such rocks routinely go back hundreds of millions of years, we have a few that go back a billion years or more. We find no epochs in our geological history where life was not also present.

But over very long durations, particularly with many layers of rock atop it, that sedimentary rock will begin to metamorphose, or change its chemical makeup. If a rock is only partially metamorphosed, it might still contain fossils, but a completely metamorphosed rock won’t have any at all. This might cause you to lose hope, concluding that once we go back beyond about ~2 billion years in Earth’s history, there won’t be any way to tell whether our planet was inhabited or not.
But there is a way.
You’ve heard of carbon-dating before, where we can use the ratios of different carbon isotopes in an to estimate how long it’s been since the remnants of organic matter have stopped undergoing biological processes. You measure the ratio of two different isotopes: carbon-12 and carbon-14. Carbon-12 is stable, but carbon-14 is created in the upper atmosphere from cosmic ray collisions. When you live, you breathe and ingest both forms of carbon; when you die, carbon-14 decays (with a half-life of around 5,700 years) and is not replaced. Thus, when you measure that ratio, you can tell how long ago a particular organism died, up to perhaps 100,000 years ago or so.
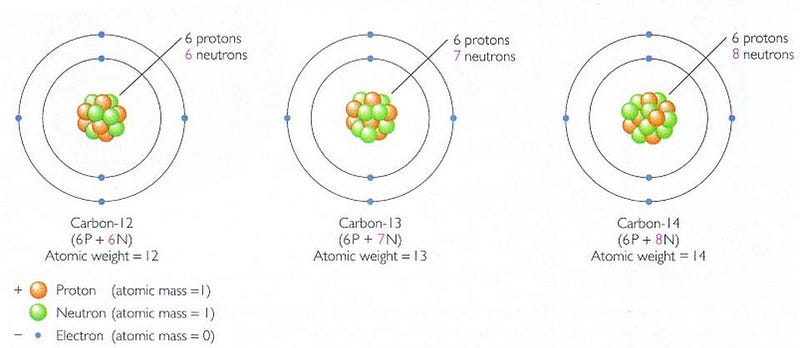
But there’s another form of carbon: carbon-13, which is stable like carbon-12, and makes up about 1.1% of the carbon found on Earth. Living organisms — at least, to the best of our understanding — preferentially uptake carbon-12 versus carbon-13, and we see a reason why when we look at the metabolic activity of enzymes: they’re more reactive with molecules that contain carbon-12 than carbon-13.
When you look at an ancient source of carbon, you can be pretty sure that if it has the standard (1.1%) amount of carbon-13 in it, it probably arose from an inorganic process. but if it has less carbon-13 and a relative enhancement of carbon-12, that’s a good indication that you’ve found the remnant of an organic life form.
When scientists search for ancient remnants of life, they look for graphite deposited in highly metamorphosed rocks. This method led us to push the emergence of life, based on evidence from Earth-based rocks, back to 3.8 billion years ago, or just 750 million years after Earth formed. But looking at graphite deposits in zircons — some of which are 4.1 billion years old or possibly even older — shows this same carbon-12 enhancement at the expense of carbon-13.
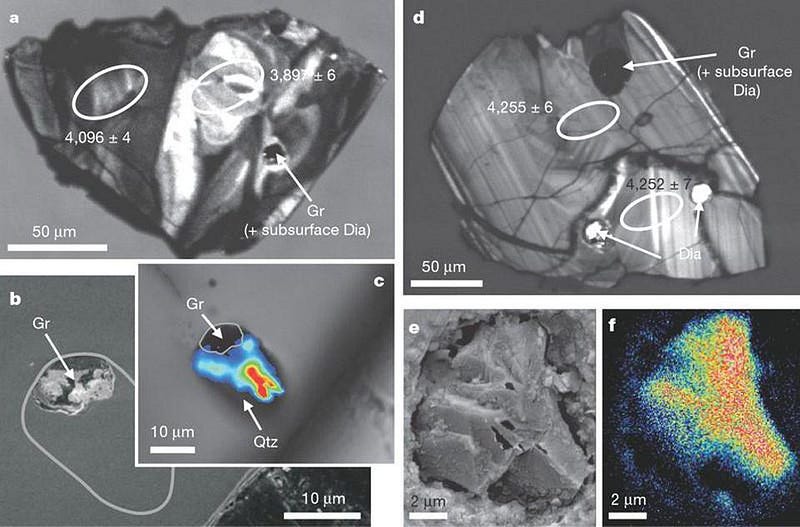
This tells us, at the very least, that life on Earth very likely goes back a very long time: to when Earth was less than 10% its current age. Most have assumed this implies that life arose very early on in Earth’s history, perhaps even during its most primordial stages. But there’s another possibility that’s even more fascinating: perhaps the life that we find on Earth didn’t originate on Earth, but was formed prior to it.
Perhaps, once Earth formed, there were extraordinarily primitive organisms that came to Earth, found they could survive and reproduce here, and that’s how life began on our planet. As wacky and wild as this idea sounds, it’s a hypothesis that we not only cannot rule out, but one that has a wide variety of indirect support that bolsters its plausibility.
The idea that Earth was “born” with life already on it really, truly could be the case. Here’s why this is a scientifically interesting scenario to explore.
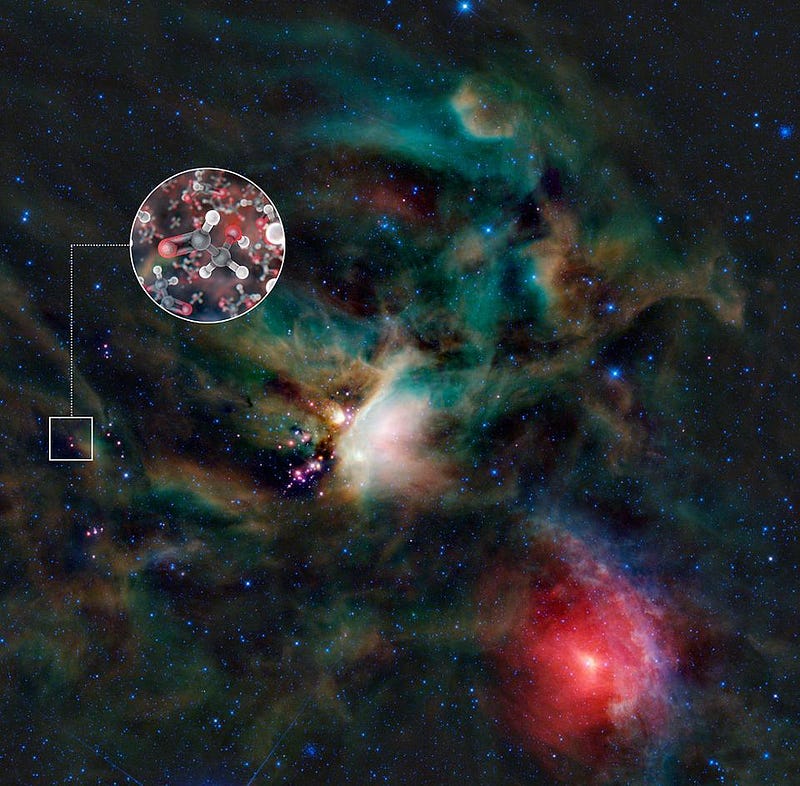
Reason #1: time and ingredients are abundant. Although Earth formed 4.5 billion years ago, the Universe was around, doing its thing, for over 9 billion years before that. Stars lived, burned through their fuel, and died in both supernovae and planetary nebulae: recycling heavy elements into material that would form new stars. Neutron stars and white dwarfs merged, further enriching the interstellar medium. And when new stars form, they create enormous numbers of small fragments — asteroids, planetesimals, and frozen, icy bodies — many of which get ejected and travel throughout the galaxy, where their material can wind up on planets in other Solar Systems.
Given the enormous amounts of cosmic time that have passed, and how many different stars and star systems have existed throughout our galaxy’s history, there’s the tremendous potential for ingredients from one corner of the Milky Way to enrich (or infect, depending on your perspective) any other. All we needed was for life to have arisen once, somewhere, long ago, and that could provide for an origin to life on an innumerable number of subsequent worlds.

Reason #2: the precursors to life are everywhere. It’s true: we’ve never yet demonstrated how life arose from non-life here on Earth. No laboratory experiment we’ve ever done has begun with completely non-living ingredients and ended with what we would unambiguously call life. And yet, the Universe gives us tremendous hints that life as we understand it definitively originated from non-living precursors.
The clues come in many forms. Organic molecules — sugars, amino acids, and complex carbon rings — are found ubiquitously in interstellar space and in outflows around young stars. Dying stars exhibit many complex molecules, including polycyclic aromatic hydrocarbons and ethyl formate: the molecule that gives raspberries their scent. Even meteorites that have fallen to Earth, like the Murchison meteorite that struck Australia in the 1960s, contain not only all 20 amino acids found in organic processes on Earth, but more than 60 others, including many with the opposite “handedness” to the ones we use. The precursor ingredients to life are literally everywhere; all they needed was the right set of conditions to create life.
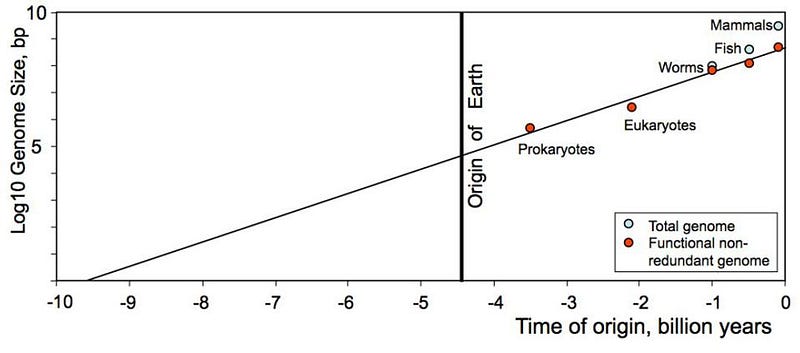
Reason #3: the complexity of life on Earth indicates, via extrapolation, a much earlier origin than Earth alone can provide. Here’s a fascinating and suggestive idea: take the most genetically complex organisms that exist today, and sequence their DNA. Take note of the length of their nucleic acid sequence, including the unique, non-overlapping genes, proteins, and other information that’s encoded in them. Then, going back through the fossil record, try and trace how that complexity has evolved. (I promise, this isn’t a creationist trick!)
What you’ll find is that the most complex organism thought to exist at any point in our history follows the pattern of growth you see above. If you go back only to the origin of Earth, you have a complexity that’s very hard to imagine from random chance: some ~30,000 base pairs in your genetic sequence. But if you go back a few billion years further — i.e., to a pre-Earth origin of life — random chance could easily account for such a seed. Perhaps we only need to investigate the interstellar medium to find evidence of the earliest life.

Reason #4: material on rocky planets doesn’t stay sequestered. The Universe might be mostly empty space, but on long enough timescales, these finite-sized objects will inevitably experience collisions with one another. Asteroids, comets, planetesimals and more smash into major bodies like planets, and with enough energy, can kick enormous amounts of debris — once part of the planet’s surface — into space. This debris can form moons, rings, can fall back to the planet, or can travel throughout the Solar System and beyond. This isn’t just conjecture; we’ve collected the evidence for meteorites from other worlds, including the Moon and Mars, that have made it to Earth.
In fact, below, you can see the Allan Hills 84001 meteorite, discovered in 1984, which is now known to have originated from Mars. In fact, 3% of all meteorites on Earth are of Martian origin. Given that both Mars and Earth have been struck by large numbers of meteorites, it’s eminently plausible that there are chunks of planet Earth constant traveling through the Solar System, and many that have been ejected to travel the galaxy at large.
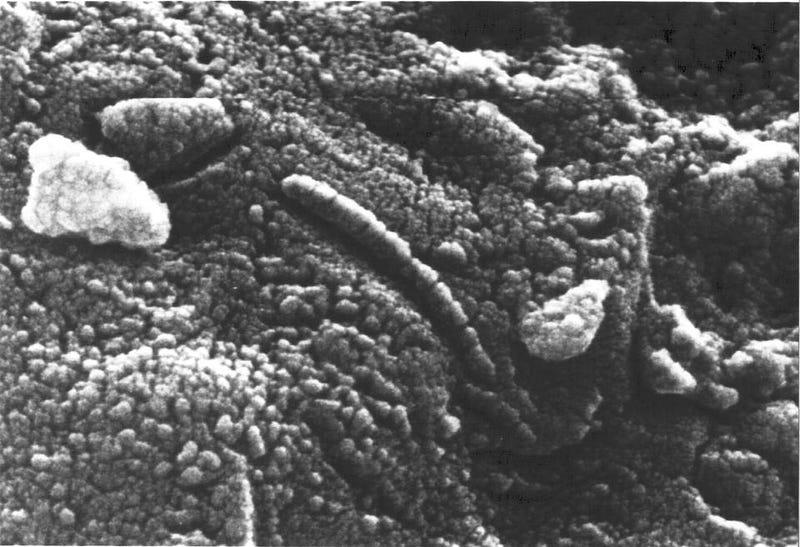
At the same time that we consider this fascinating possibility, it’s important to rein ourselves in from succumbing to our wildest imaginings. We’ve found meteorites of Martian origin with oddly-shaped inclusions in them. Although many initially jumped to the conclusion that these micron-sized shapes were fossilized Martian organisms, that was premature. Instead, we’ve found numerous inorganic processes that could lead to these inclusions. Life remains a possibility, but we need significantly stronger evidence than this dubious, ambiguous signal.
We have every indication that once life began on Earth, it continued to survive, thrive, reproduce, mutate, and evolve in an unbroken chain spanning more than 4 billion years. But despite all that our scientific investigations have uncovered, we still don’t know whether our terrestrial life originated on our planet, or in a different place at an earlier time. Furthermore, we strongly suspect that Earth life has since stowed away on collisional fragments that have journeyed throughout the Solar System, Milky Way, and possibly even beyond.
We often say “there is no planet B” out there, but that’s just for humans. Perhaps, if we could trace out the cosmic chain of life, Earth is just one link: not the first, and not the last, but an incubator of a story that began billions of years before. As with most open questions in science, until we have the decisive evidence in hand, we have no option but to keep all the viable possibilities in mind while we continue the search for the answers.
Starts With A Bang is written by Ethan Siegel, Ph.D., author of Beyond The Galaxy, and Treknology: The Science of Star Trek from Tricorders to Warp Drive.