Throwback Thursday: An Atom in the Universe
The incredible story of a single simple atom, that just happens to be in your body right now!
Image credit: Richard Crisp, via http://www.narrowbandimaging.com/incoming/horse_ap155edf_pl39k_lrgb_15hrs.jpg.
“The atoms come into my brain, dance a dance, and then go out — there are always new atoms, but always doing the same dance, remembering what the dance was yesterday.” –Richard Feynman
Here you are, a human being, a grand Universe of atoms that have organized themselves into simple monomers, assembled together into giant macromolecules, which in turn comprise the organelles that make up your cells. And here you are, a collection of around 75 trillion specialized cells, organized in such a way as to make up you.
Of those 75 trillion cells, about 4 trillion are what you think of as “you,” with about half of the rest being red blood cells and the other half being bacterial cells that are simply hosted in your body at any given time. But those cells, every last one of them, are themselves composed of trillions-to-quadrillions of atoms. At our core, atoms are all that we are. A mind-bogglingly large number of atoms — some 10^28 of them — but atoms nonetheless.
Those two things — you and an atom — may seem so different in scale and size that it’s hard to wrap your head around. Here’s a fun way to think about atoms: if you broke down a human being into all the atoms that make you up, there are about as many atoms that make up you (~10^28) as there are “a-human’s-worth-of-atoms” to make up the entire Solar System!
If you summed up the mass of all the known Solar System objects, about 99.8% of what we know is found in the Sun, with Jupiter making up 0.1% and everything else — planets, moons, asteroids, comets, etc. — making up the rest.
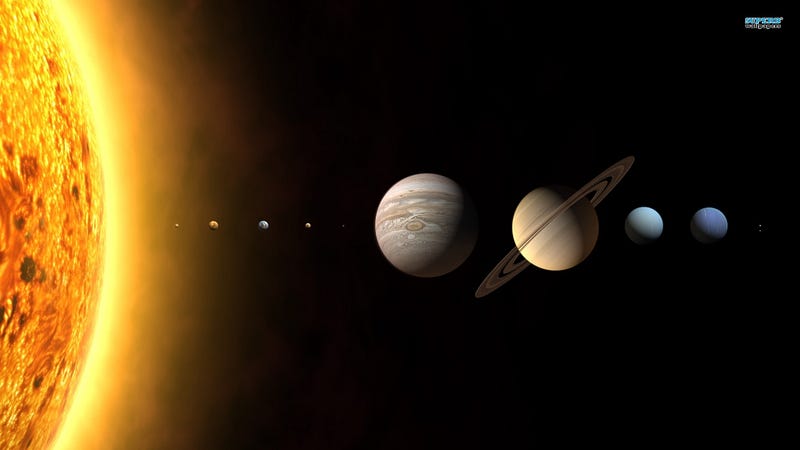
All the matter in the Solar System, all summed together, contains about 10^57 atoms, or 10^29 human-beings-worth of atoms. So an atom, compared to you, is approximately as tiny as you are in comparison to the entire Solar System, combined.
But that’s just for perspective. The 10^28 atoms that are existing-as-you-right-now each have their own story stretching back to the very birth of the Universe. Each one has its own story, and so today I bring you the story of just one atom in the Universe. In isolation, a single atom isn’t so interesting, but as part of your body, right now, I can think of nothing more important.
There was a time in the distant past — some 13.8 billion years ago — when there were no atoms. Yes, the energy was all there to make them, but it was far too hot and too dense to have even a single atom form. Imagine it if you can: all the matter in the entire Universe, some 10^91 particles, in a volume of space about equal to that of a single, giant star.
The whole Universe, compressed into a volume of space that one large star takes up.
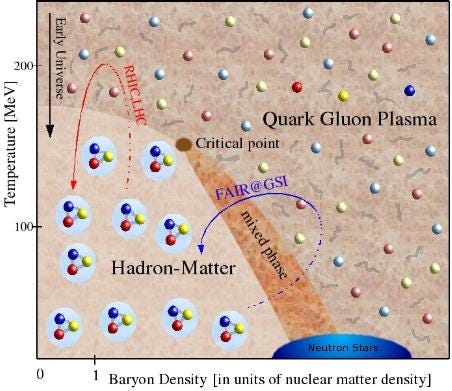
Yes, back then it was too hot to have any atoms at all. But the Universe didn’t stay that way for long: it may have been incredibly hot and dense, but it was expanding and cooling incredibly rapidly back then. After less than a second, the quarks and gluons had condensed into stable protons and neutrons, the building blocks of all atomic nuclei, with an almost equal amount of anti-protons and anti-neutrons.
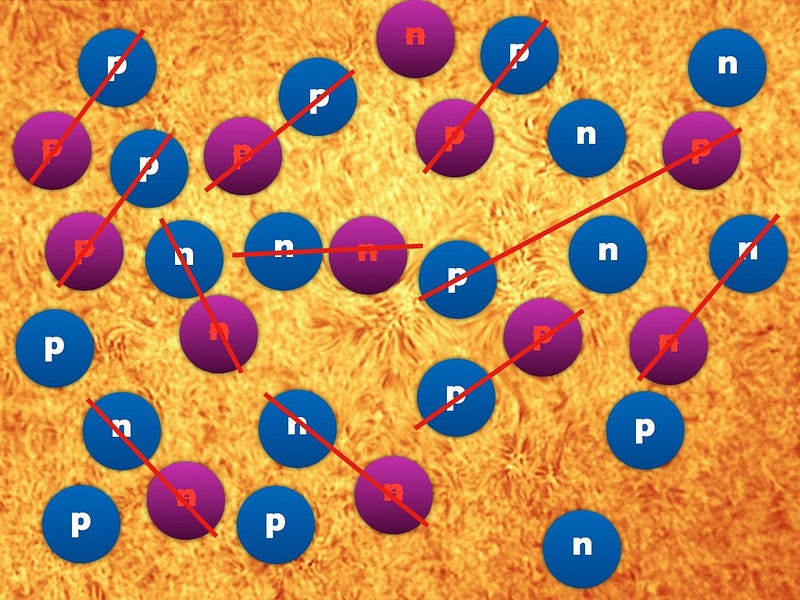
The Universe was 50.00000003% matter and 49.99999997% antimatter at that time, with some radiation and dark matter thrown in. As it continued to expand and cool, practically all of the antimatter collided with matter, annihilating away, and leaving just a tiny amount of matter — protons, neutrons and electrons — behind.
The atom we’re thinking of started out as a neutron. Protons tried to fuse with it to create deuterium, but the Universe was too hot for that to happen, and each time it formed deuterium, it was blasted apart less than a nanosecond later.
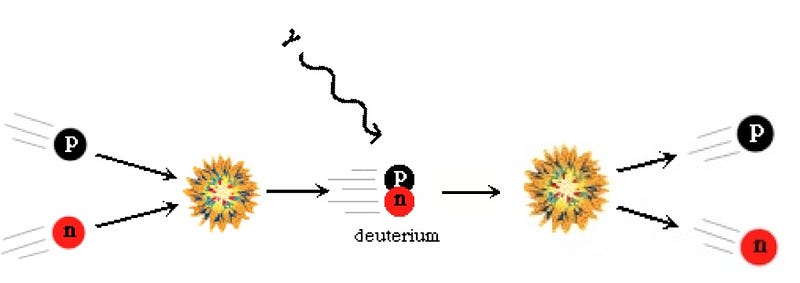
After about three minutes, a few of the free neutrons had decayed into protons, but this one remained. If it had taken thirty minutes, the majority of neutrons would have decayed away, including this one, but after only three, the Universe had cooled enough so that nuclear fusion could proceed.
The neutron quickly formed deuterium, then Helium-3, and finally found another deuteron to become a Helium-4 nucleus. Only about 8% of the atoms in the Universe became Helium-4 like this one; the other 92% were just plain old protons, also known as Hydrogen nuclei.
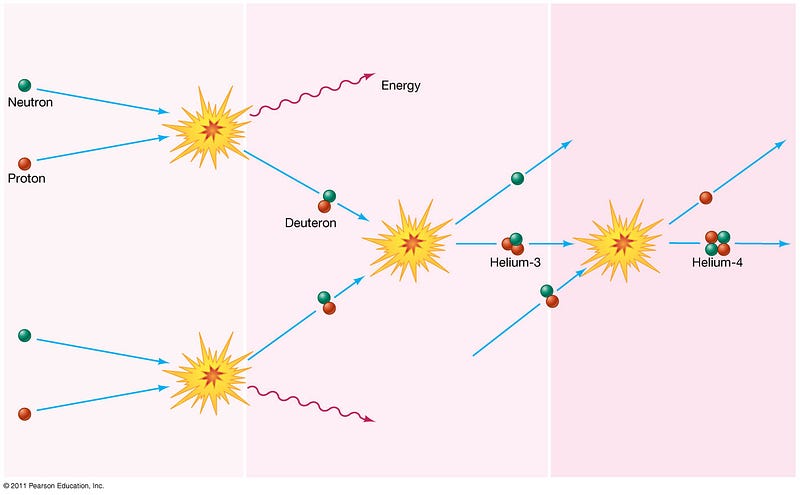
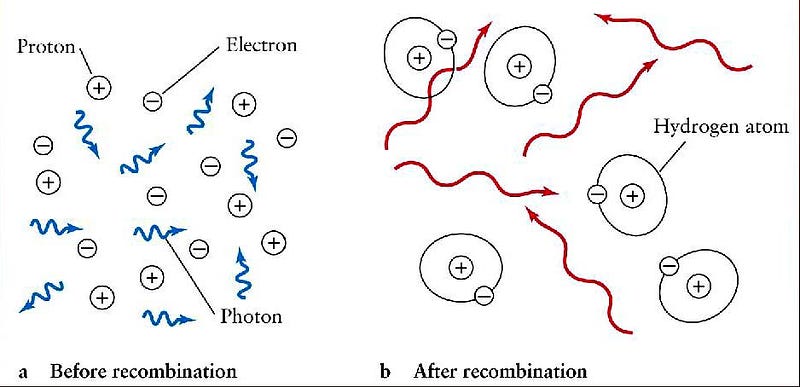
With a positive charge to it, this helium nucleus kept on trying to attract electrons, something that it did for hundreds of thousands of years, and yet it couldn’t hang on to one for more than a fraction of a second. The energetic background of radiation was too strong, and kept it ionized and isolated, while the repulsive force from other nuclei kept it from growing any larger.
It took another 380,000 years for the Universe to cool enough for this to become a neutral atom, and for two electrons to join this nucleus. The Universe — despite its rapid expansion and cooling — remained 100% ionized until the temperature dropped to just a few thousand degrees, which simply took that much time.
Over the next hundred-million years or so, this atom found itself caught up in the gravitational pull of the Universe, which began to collapse the overdense regions of matter into clumps. These clumps would eventually form stars and galaxies from the hydrogen and helium left over from the early Universe. But the vast majority of atoms — more than 95% — weren’t a part of the first generation of stars, and neither was this one in particular.
Instead, when the first stars formed, they kicked the electrons out of the atoms that surrounded them, creating ions once again.

It was only by luck that this atom we’re following wound up in a dense molecular cloud, far away from the ionizing ultraviolet radiation from the hottest of the young stars. After more than a billion years in this collection of neutral atoms, the molecular cloud housing this atom began an irresistible gravitational collapse of its own. Our atom finally found itself pulled in by gravitational attraction to what would become a giant, blue star.

This atom lost its electrons and fell to the core of the star, where it lay dormant for millions of years, as hydrogen nuclei fused into other helium nuclei just like this one. Despite the tremendous nuclear furnace at this star’s core, however, this helium atom, like all the helium atoms in the star’s core, remained untouched.
Only when the core ran out of hydrogen fuel did helium fusion begin, and in short order, our atom fused with two others to become a carbon nucleus!

While other atoms even closer to the center of the star fused further, carbon was as far as this particular atom went. When the core of the star collapsed and the star went supernova, our atom was blown out into the interstellar medium, where it remained for billions of years.

While billions of other stars in our Milky Way alone went through the life-and-death cycle, this carbon atom remained in interstellar space, eventually picking up six electrons to become neutral.
It found its way into a gravitational collection of neutral gas, a molecular cloud that was much smaller than the previous one that its progenitor helium atom was found in. Over time, this neutral gas cloud cooled, and eventually our atom found itself getting sucked in to another gravitational perturbation, as star-formation happened all over again.

This time, the atom didn’t find its way into the central star of its system, but rather into the dusty disk that surrounded it. Over time, the disk separated into planetoids and planetesimals, and this atom found itself aboard one of those.
By chance, it happened to be something of a Johnny-come-lately to its planetoid, and wound up joining up with four hydrogen atoms — becoming a methane molecule — in the atmosphere of this newly-formed world.
As various energetic events took place, ranging from sunshine to lightning storms to meteor strikes to chemical diffusion, this methane molecule (and the carbon atom at its core) went through millions of different chemical reactions over time.
After life took hold on Earth, it came in-and-out of organic situations many times. At one point it became a part of a bacterium’s DNA, then at a later time a part of a plant’s cell wall, and eventually it became part of a complex organism that would find itself consumed by you.

The atom is currently in a red blood cell of yours, where it will remain for a total of about 120 days, until the cell is destroyed and replaced by a different one.
Although the cell — and all cells in your body — will be destroyed and replaced, you will remain the same person you are, and the atom will simply take on a different function, whether in your body or out of it.
The atoms in your body are temporary, and can all be replaced — unnoticed by you — by another of the same type. In fact, every atom in your body cycles out of your body after about six years at most.
Yet somehow, for reasons that we don’t completely understand, you still remain you, even though all your atoms may be different. And each and every one of the 10^28 atoms in your body has a story as spectacular and unique as this one! As Feynman once poetically said,
“I
a Universe of atoms
an atom in the Universe.”
The story of the Universe is inside every atom in your body, each and every one. And after 13.8 billion years, 10,000,000,000,000,000,000,000,000,000 of them have come together, and that’s you. The Universe is inside of you, as surely as you’re inside the Universe.

You, a Universe of atoms, an atom in this Universe.
Have something to say? Say it at the Starts With A Bang forum on Scienceblogs!