Surprise: The Third Most Common Element In The Universe Isn’t What You Think
After hydrogen and helium, the periodic table is full of surprises.
“The two most common elements in the universe are hydrogen and stupidity.” –Harlan Ellison
One of the most remarkable facts of existence is that every material we’ve ever touched, seen, or interacted with is made up of the same two things: atomic nuclei, which are positively charged, and electrons, which are negatively charged. The way these atoms interact with each other — the ways they push-and-pull against each other, bond together, attract and repel, and create new, stable molecules, ions, and electron energy states — is literally responsible for the entirety of the world around us.
Even though it’s the quantum and electromagnetic properties of these atoms and their constituents that enable our Universe to exist with the properties we observe, it’s important to realize that the Universe didn’t start out with all the ingredients necessary to create what we know today. Quite to the contrary, it started out with hardly any of them.

You see, in order to achieve these various bond structures, and to build complex molecules which make up the building blocks of all we perceive, we needed a huge variety of atoms. Not just a large number of atoms, mind you, but atoms that show a great diversity in type, which means atoms with varying numbers of protons present in their atomic nucleus: the very thing which makes different elements.
Our very bodies themselves require elements like carbon, nitrogen, oxygen, phosphorous, calcium and iron. The crust of our Earth itself requires silicon and a myriad of other heavy elements, while the Earth’s core — in order to generate all of its heat — requires elements going all the way up the periodic table to the heaviest naturally occurring ones we find: thorium, radium, uranium, and even trace amounts of plutonium.
But back in the very early stages of the Universe — before humans, before there was life, before there was our Solar System, before there were rocky planets or even the very first stars — all we had was a hot, ionized sea of protons, neutrons and electrons. There were no elements, no atoms, and no atomic nuclei: the Universe was too hot for any of that. It was only because the Universe expanded and cooled that we were able to form anything stable at all.
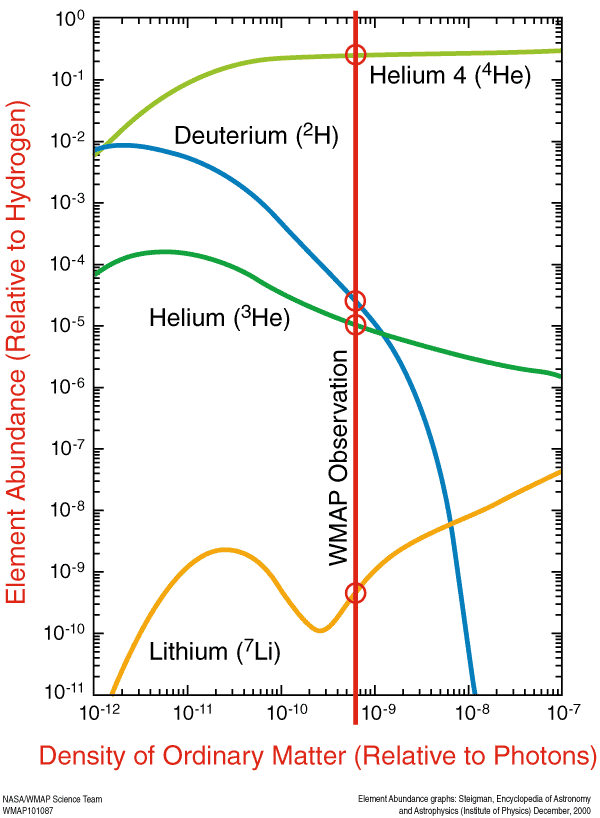
But time passed, and we did. The first nuclei fused together without immediately being blasted apart, producing hydrogen and its isotopes, helium and its isotopes, and tiny, trace amounts of lithium and beryllium, the latter of which would radioactively decay into lithium. This is the Universe we started off with: a Universe that was — by number of nuclei — about 92% hydrogen, 8% helium, and about 0.00000001% lithium. By mass, that’s about 75–76% hydrogen, 24–25% helium, and 0.00000007% lithium. Pretty much all hydrogen and helium, any way you slice it.
Hundreds of thousands of years later, the Universe cooled enough that neutral atoms could form, and then tens of millions of years after that, gravitational collapse enabled the first stars to form. And with that, the phenomenon of nuclear fusion not only brought light back to the Universe, but brought heavy elements to our reality as well.
The moment the first star is born, some 50-to-100 million years after the Big Bang, copious amounts of hydrogen start fusing into helium. But even more importantly, the most massive stars (the ones more than about 8 times as massive as our Sun) burn through that fuel very quickly, in just a few million years themselves. Once they run out of hydrogen in their cores, that helium core contracts down and starts fusing three helium nuclei into carbon! It only takes approximately a trillion (10¹²) of these heavy stars existing in the entire Universe (which forms about 10²² stars in the first few hundred million years) for lithium to be defeated.

But will it be carbon that breaks the record, and comes in at element #3 today? You might think so, since stars fuse elements in onion-like layers. Helium fuses into carbon, then at higher temperatures (and later times), carbon fuses into oxygen, oxygen fuses into silicon and sulphur, and silicon finally fuses into iron. At the very end of the chain, iron can fuse into nothing else, so the core implodes and the star goes supernova.
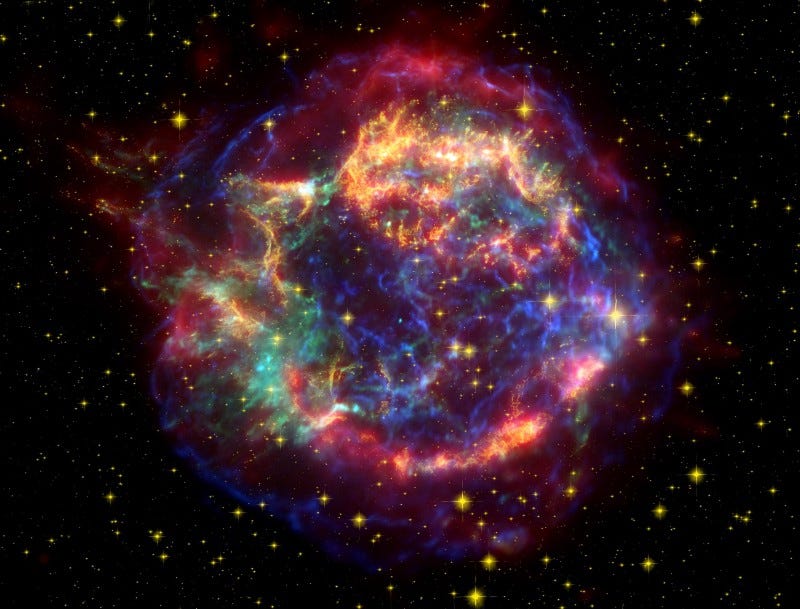
These supernovae, the steps leading up to them and even their aftermaths, enrich the Universe with all the outer layers of the star, which returns hydrogen, helium, carbon, oxygen, silicon, and all the heavier elements formed through a few other processes:
- slow neutron capture (the s-process), building elements up sequentially,
- the fusion of helium nuclei with heavier elements (creating neon, magnesium, argon, calcium, and so on), and
- fast neutron capture (the r-process), creating elements all the way up to uranium and even beyond.
But we don’t even have just this single generation of stars: we have many, and the ones that exist today are primarily built out of not only the pristine hydrogen and helium, but the leftovers from previous generations. This is important, because without that, we’d never get rocky planets, only gas giants of hydrogen and helium, exclusively!

Over billions of years, the process of star formation and star death repeats itself, although with progressively more and more enriched ingredients. Now, instead of simply fusing hydrogen into helium, massive stars fuse hydrogen in what’s known as the C-N-O cycle, leveling out the amounts of carbon and oxygen (with somewhat less nitrogen) over time.
Additionally, when stars undergo helium fusion to create carbon, it’s very easy to get an extra helium atom in there to form oxygen (and to even add another helium to the oxygen to form neon), something even our paltry Sun will do during the red giant phase.
But there’s one killer move that stars have that makes carbon a loser in the cosmic equation: when a star is massive enough to initiate carbon fusion — a requirement for generating a type II supernova — the process that turns carbon into oxygen goes almost to full completion, creating significantly more oxygen than carbon by time the star is ready to explode.
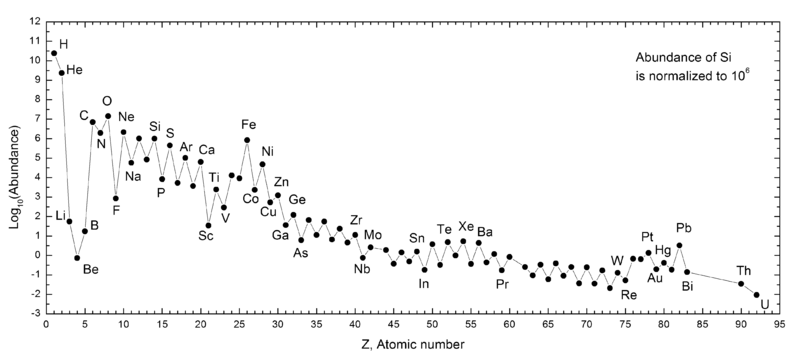
When we look at supernova remnants and planetary nebulae — the remnants of very massive stars and sun-like stars, respectively — we find that oxygen outmasses and outnumbers carbon in each and every case. We also find that none of the other, heavier elements come close!
Yes, hydrogen is still #1 by a wide margin, and helium is #2 by a very large amount as well. But of the remaining elements, oxygen is a strong #3, followed by carbon at #4, then neon at #5, nitrogen at #6, magnesium at #7, silicon at #8, iron at #9, and sulphur rounding out the top 10.
What will the far future hold?
Over long enough time periods, periods that are at least thousands (and probably more like millions) of times the present age of the Universe, stars will continue to form until the fuel is either ejected into intergalactic space, or until its completely burned as far as it can go. When this occurs, helium might finally overtake hydrogen as the most abundant element, or hydrogen may stay #1 if enough of it remains isolated from fusion reactions. On extraordinary long timescales, the matter that doesn’t get ejected from our galaxy may wind up fusing together, over and over, so that carbon and oxygen might wind up someday surpassing even helium; perhaps our current #3 and #4 may wind up cracking the top two?

The most important thing is to stick around, because the Universe is still changing! Oxygen is the third most abundant element in the Universe today, and in the very, very far future, may even have the opportunity to rise further as hydrogen (and then possibly helium) falls from its perch. Every time you breathe in and feel satisfied, thank all the stars that lived before us: they’re the only reason we have oxygen at all!
Leave your comments on our forum, help Starts With A Bang! deliver more rewards on Patreon, and pre-order our first book, Beyond The Galaxy, today!