Science, And Its Limitations, Showcase The Need For Earth Day

Science can teach us so much about our planet, but something more must compel us to take care of it.
If you want to understand our planet, the best way to go about it is scientifically: by asking the Earth questions about itself. Through the process of careful observation, measurement, and even experimentation, we can learn how the planet — and everything on and within it — responds under a wide variety of conditions. We can also observe other planets, other star systems in various stages of formation and evolution, and objects in interstellar space itself, to better piece together the behavior of our home world.
From the outermost reaches of Earth’s atmosphere all the way down into the center of our core, our studies have revealed a tremendous amount of information about our planet. From the thin biosphere, teeming with life, going both way out into space and way down into the interior, Earth is full of physics, chemistry, geology and biology to marvel at. But unless, as humans, we band together to take collective action to responsibly steward our planet for future generations, we’ll wind up creating a future riddled with disaster for our descendants to reckon with. Here’s why we need Earth Day.

As far as we can tell, Earth formed just like every other planet: from a collapsing cloud of molecular gas that fragmented to form new stars. When these interstellar gas clouds grow large enough, they gravitationally contract, radiating excess energy away primarily through heavy elements and bound molecules. If they can cool successfully, the largest masses within them will gravitationally grow relatively quickly, heating up and forming proto-stars.
Surrounding these proto-stars are large disks of material: mostly hydrogen and volatile molecules, but with a small but substantial fraction of heavier elements in there. Due to a combination of factors — pressure, radiation, high-energy particles emitted from the proto-star, etc. — the lighter elements closest to the proto-star get expelled, leaving primarily denser elements there.
After a few tens of millions of years, we wind up with a system of planets, along with an asteroid belt at the old “frost line” and a series of smaller, icy bodies in a disk and then a cloud out beyond the final planet.
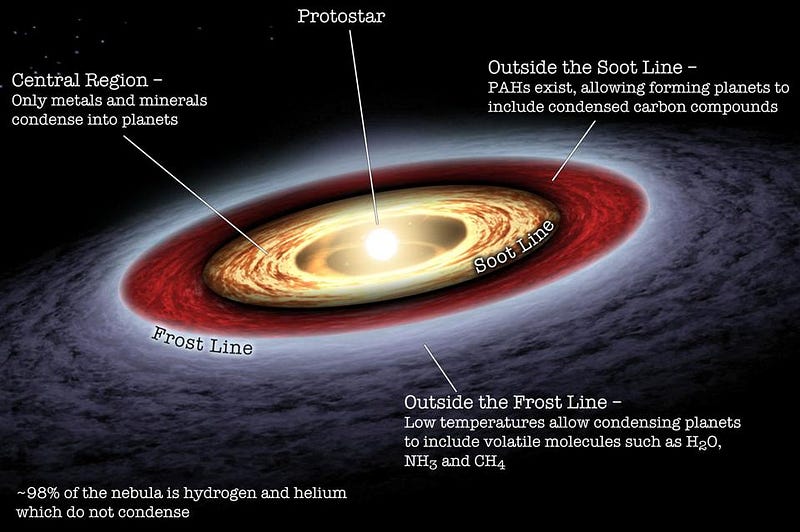
Although the mass distribution of planets in our Solar System may not be the most common way the Universe arranges its planets, we don’t quite believe we’re far from typical. Rather, a number of things happened in Earth’s early history that we have indications are quite common, including the following.
- A large, early collision with a major planetesimal created a cloud of debris — a synestia — that gave rise to our Moon; we believe similar collisions occurred on Mars, creating three moons (that are now down to two), as well as Pluto, creating its lunar system.
- The surface of the planet, initially devoid of volatiles (as they were likely blown off by the newly-forming Sun), collected material similar to that found in our outer Solar System, bringing water and other surface elements to our world, a process we believe occurs for most planets.
- And the raw ingredients for life, so common not only on our world but throughout the Solar System and galaxy, are found ubiquitously on the surface. Not only heavy elements, but many of the chemical compounds necessary for life (amino acids, sugars, carbon-ringed molecules, cyanides, etc.) are found all throughout the Universe.

Although life successfully took hold on Earth from at least a very early time — it’s been present for more than 90% of our planet’s history — we believe it only exists in a thin shell on, above, and just slightly beneath Earth’s surface. Our biosphere, though it covers the surface area of Earth and extends all the way down to the ocean bottoms, beneath the surface and into the crust, and well up into the atmosphere, represents only a tiny fraction of the full volume of Earth.
Beneath our feet, an incredible array of processes are constantly taking place. In the early stages of our planet’s history, back when the Earth was first forming, the lightest, lowest-density, most buoyant elements were driven away from Earth’s center, while the heaviest, densest elements sank to the core. A tremendous amount of heat, left over from the formation of the planets in the Solar System and from gravitational contraction, became trapped within our planet, while the radioactive elements present throughout Earth started to decay.
Over the history of our planet, gravitational contraction and radioactive decay each contribute about half of our planet’s internal energy, while the external energy received is overwhelmingly dominated by the Sun.

This internal energy — which we sometimes attempt to harness as geothermal energy — leads to some surprising facts. As we dig into the Earth, even in regions where there aren’t magma chambers nearby or a history of volcanic activity, the temperature gradually but swiftly increases. The increase in heat that we encounter is heavily responsible for limiting our attempts to drill beneath Earth’s crust and into the mantle; despite the fact that we’ve drilled thousands of meters down beneath the surface, requiring that we break into the bedrock, we haven’t come close due to the heat.
If we could, however, we’d see that temperatures increase extremely rapidly. Every few hundred meters, the temperature rises by a full degree Celsius. By the time we get somewhere between about 0.5%-to-1% of the way to the Earth’s core, corresponding to only a few dozen kilometers, the Earth itself will no longer be dark. At a temperature of around 500 °C (a little over 900 °F), the Earth itself gets so hot that it will start to glow in visible light, appearing a dull brownish-red from blackbody radiation.
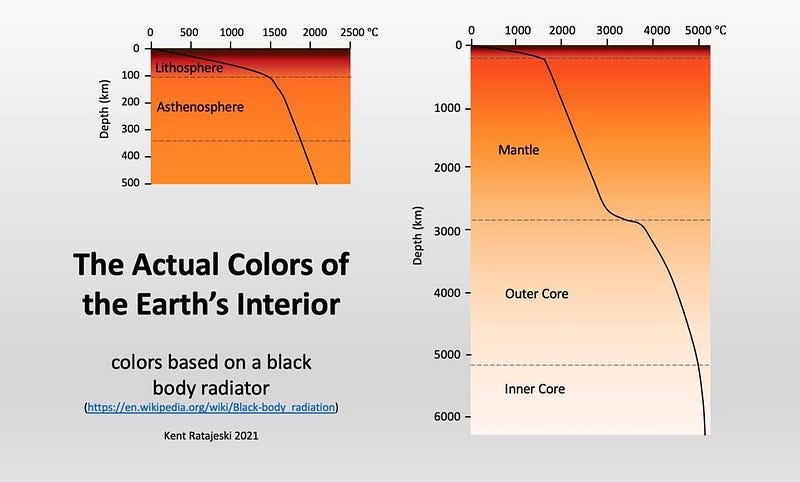
But this is just the start of what goes on in the interior of Earth. As we further descend into the Earth’s mantle, the temperature heats rapidly. At ~660 °C, certain softer, common metals, like lead, will melt. At ~1300 °C, iron and steel melt as well. But not everything that we encounter, once we exceed these temperatures, becomes liquid. Another factor is also at play: beneath the surface of the Earth, the pressure increases very rapidly. As the pressure goes up, certain materials are far more likely to be found in solid form, rather than liquid or any other.
In fact, once you get below the crust/mantle boundary, where the magma chambers that lead to volcanoes and deep sea vents are frequently found, the Earth is not only largely solid, but far denser than the rocky material found in the crust. The deeper we go, the more the density increases. As far as we can tell, the Earth’s mantle makes up the majority of our planet — by volume and by mass — and then transitions to a liquid state: where the outer core is.
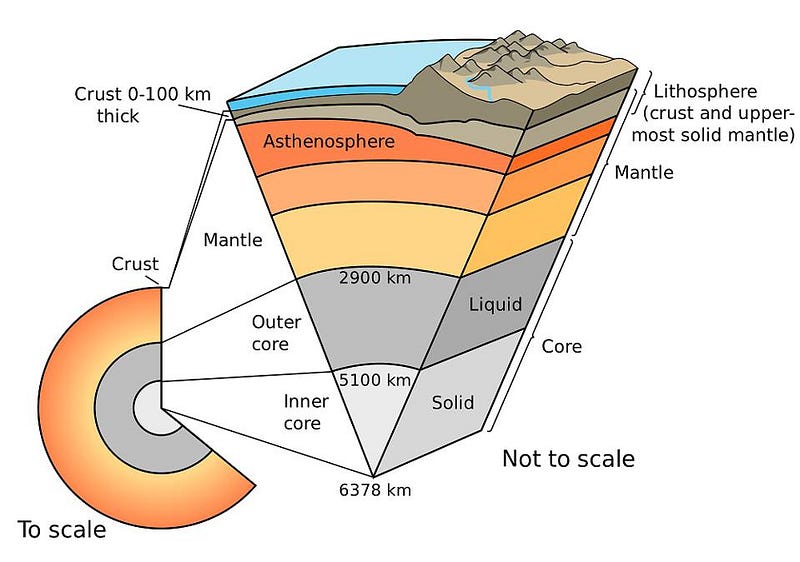
This liquid outer core was discovered seismically: by examining the way that earthquakes travel through our planet to be “felt” at different locations on the surface. Whenever you have a phase transition — from solid to liquid or liquid to solid, for instance — you will observe these waves “bend” as the material they pass through changes, the same way the light from a pencil or straw immersed in a glass of water appears to “bend” when you view it from the side.
If we go to the deepest insides of the Earth, to the inner core, things go back to solid again. This is the densest, hottest, most extremely pressurized part of the Earth, with temperatures exceeding 5000 °C: making the Earth’s center almost as hot (and causing it to glow almost as white) as the surface of the Sun. Although the inner core is “only” about ~750 km in radius, representing about ~12% of the Earth, it was recently discovered that the inner core itself may consist of two separate layers, dividing our planet into five components, rather than the traditional four.

We’ve also traveled in the opposite direction: far away from our planet, enabling us to view it from great distances. From about ~40 kilometers up, the height that balloons can routinely reach, we can see and measure the curvature of Earth. From the height of the International Space Station — stably in low-Earth orbit — we can circle the globe in just 90 minutes. And from farther away, as we break away from the gravitational bonds of our planet, we can even see the entire spheroid of Earth all at once, and watch it rotate about its axis in real time.
We’ve obtained even more distant views as well. We’ve viewed Earth through the lens of many of our different spacecraft visiting many different planets. We’ve looked back at Earth from the Moon, from Mercury, from Mars, from Jupiter and Saturn, and even from out beyond the final planet in our Solar System. Our views of Earth from space are iconic, reminding us how small, fragile, and precious our world is. For any question we may have about the physical nature of our planet, the appropriate scientific investigations can reveal extraordinarily accurate answers.
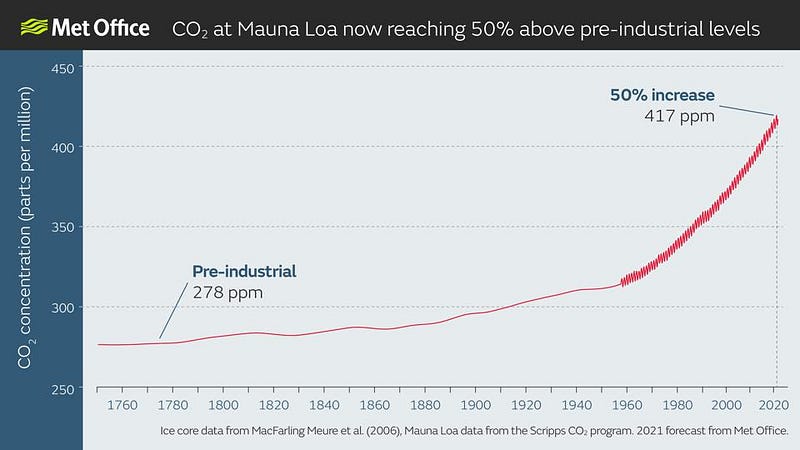
But what science cannot do, on its own, is spur us to collective action. We can track how our planet is changing — how it’s changed over the course of its natural history, as well as how it’s changed due to the recent influences of human civilization — and science can inform us on that front. For example, it can tell us:
- how human civilization has driven changes in our atmosphere’s contents,
- the degree of acidification that’s occurred in Earth’s oceans over the past ~200 years,
- at what rate the planet is warming and ocean levels are rising,
- what the estimated rate of species extinction is at present, and how that compares to historic levels,
- and how these — and other factors — will continue to evolve into the future if various plausible scenarios play out in a large assortment of ways.
The part that’s up to us, however, goes far beyond what science tells us: what are we going to do about it? Science can tell us what certain likely outcomes are for certain paths of actions and inactions, but it cannot compel us to be good stewards of the planet. Science can point the way towards a responsible future, but it’s up to us, collectively, to make that our reality.

51 years after the inception of Earth Day, humanity finds itself on the brink of a new era. With the Earth warming, sea levels rising, the climate changing, and our atmospheric concentration of greenhouse gases — the driving factor behind it all — now increasing at a faster rate than ever before, the next few decades will be critical, and will impact the Earth significantly for millennia to come.
Will we take drastic measures to reduce our carbon emissions, or will we blow past unprecedented CO2 milestones: 500, 600, even 1000 parts-per-million?
Will we reorganize the way humans live and produce food and power, effectively re-wilding the Earth, or will we continue to remove our natural, wild places until the planet suffers various forms of ecological collapse?
Will we attempt a variety of geoengineering solutions to climate change, such as blocking out sunlight or seeding clouds in the atmosphere, and if so, what unforeseen consequences will they have?
Or will we do nothing, and simply resign ourselves to a future where nature will do its worst, with the climate changing swiftly and dramatically in an unabated fashion?
In all the known Universe, there’s no evidence that any other force will save us from ourselves. This is the only inhabited planet known, and the cost of terraforming any other world is far, far greater than the cost to maintain Earth’s ideal habitability for humans.
Today, moreso than any other day, let’s remember to think about something bigger than ourselves. Let’s think about the one planet that gave rise to us all, and that innumerable future generations of humans will someday call home. Let’s think about the Earth as a whole, and let’s do the best job we can of passing it on to our descendants in a better fashion than we found it.
Starts With A Bang is written by Ethan Siegel, Ph.D., author of Beyond The Galaxy, and Treknology: The Science of Star Trek from Tricorders to Warp Drive.