Photosynthesis is nearly 100% efficient. A quantum experiment shows why
- In physics, a system is 100% efficient if it can use 100% of the energy inputted to perform some type of energy-intensive work.
- In plants, nearly 100% of the incident photon energy from the Sun gets converted into electron energy that eventually powers the production of sugar: the photosynthetic process.
- Despite the fact that plants are not regularly ordered systems and that photon energy comes in a broad distribution, photosynthesis is nearly 100% efficient. Here’s how quantum physics does it.
In terms of energy, the “holy grail” of any physical system is 100% efficiency. It’s a near-impossible goal under most conditions, as from the moment any form of energy first gets transferred into a system, it inevitably gets lost to a variety of factors — heat, collisions, chemical reactions, etc. — before finally accomplishing the ultimate task it was designed for. The only ways that physicists have managed to create systems with near-perfect efficiency is to push nature to its extremes:
- at temperatures near absolute zero,
- by firing monochromatic (laser) photons at (crystalline) systems with absorptive lattices,
- or under extreme circumstances such as superconductivity and superfluidity.
But nature has provided us with a very surprising exception to that rule: plants. The humble plant, along with other, more primitive photosynthetic organisms (like certain species of bacteria and protists), absorbs a fraction of sunlight at specific (blue and red) wavelengths to convert that light (photon) energy into sugars via the complex process of photosynthesis. Yet somehow, despite obeying none of the above physical conditions, nearly 100% of that absorbed energy gets converted into electron energy, which then creates those sugars via photosynthesis. For as long as we’ve known about the underlying chemical pathway of photosynthesis, this has been an unsolved problem. But thanks to the interface of quantum physics, chemistry, and biology, we may finally have the answer, and biological disorder is the key.
It’s very important, whenever a scientist talks about “efficiency,” to recognize that there are two different definitions that are used, depending on which scientist is talking about it.
- Efficiency can mean examining the total amount of energy that comes out of a reaction as a fraction of the total energy that was inputted into a system. This is a definition commonly used when considering the overall efficiency of a complete, end-to-end system, holistically.
- Or efficiency can mean examining one isolated part of a system: the portion of inputted energy that’s involved in the reaction being considered, and then what fraction of that energy either gets used or gets liberated from that reaction. This is more commonly used when considering a single component of an end-to-end interaction.
The difference between that first and second definition is why two different physicists could look at last year’s tremendous fusion energy breakthrough at the National Ignition Facility and reach claims that seem at odds: that we’ve simultaneously surpassed the breakeven point for fusion energy and that nuclear fusion still uses 130 times more energy than it produces. The first is true if you consider the energy incident on a hydrogen pellet as compared to the energy liberated from the reaction, while the second is true if you consider the entire, complete apparatus, including the inefficient charging of the capacitor banks that produce the incident energy.
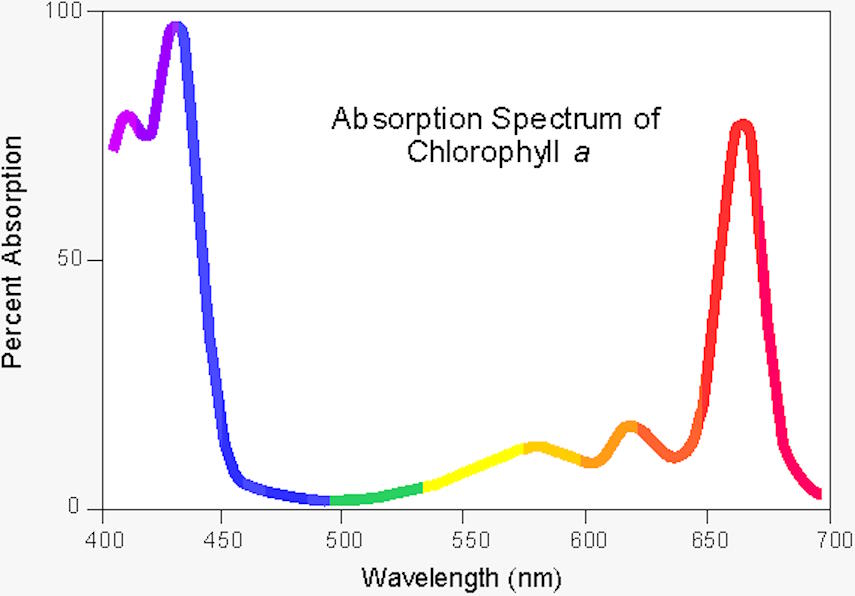
It’s true that, from a holistic viewpoint, plants are less efficient than even solar panels, which can convert somewhere around 15-20% of the total incident solar energy into electrical energy. The chlorophyll found in plants — and particularly the chlorophyll a molecule — is only capable of absorbing and using sunlight over two particular narrow wavelength ranges: blue light that peaks at around 430 nanometers in wavelength and red light that peaks around 662 nanometers in wavelength. Chlorophyll a is the molecule that makes photosynthesis possible, and is found in all photosynthetic organisms: plants, algae, and cyanobacteria among them. (Chlorophyll b, another light-absorbing and photosynthesizing molecule found only in some photosynthetic organisms, has a different set of wavelength peaks.)
When one considers all of the incident sunlight on a plant, combined, the amount of radiation that can be converted into useful energy for the plant is only a few percent of the total energy from sunlight that strikes a plant; in that strict sense, photosynthesis isn’t particularly efficient. But if we restrict ourselves to looking at only the individual photons that can excite the chlorophyll a molecule — photons at or near the two absorption peaks of chlorophyll a — the red-wavelength photons are around 80% efficient, while the blue-wavelength photons are over 95% efficient: close to that perfect, 100% efficiency after all.
This is where the big puzzle arises. Let’s walk through the steps that occur.
- The light that gets absorbed by a chlorophyll molecule isn’t monochromatic, but rather the light that gets absorbed is made of individual photons that possess a rather wide range of energies.
- Those photons excite electrons within the chlorophyll molecule, and then when the electrons de-excite, they emit photons: again, over a range of energies.
- Those photons are then absorbed by a series of proteins — where they excite the electrons within the protein, the electrons then spontaneously de-excite, re-emitting photons — until those photons are successfully shepherded to what’s known as the photosynthetic reaction center.
- Then, when the photon strikes the photosynthetic reaction center, cells convert that photon energy into electron energy, and those energetic electrons are then used in the photosynthetic process that eventually leads to the production of sugar molecules.
That’s a broad-level overview of what the pathway for photosynthesis looks like, from the relevant incident photons to the energetic electrons that wind up creating sugars.
The puzzle in all of this is why, for every photon that gets absorbed in that very first step, approximately 100% of those photons wind up producing excited electrons at the end of the last step? In terms of efficiency, there are really no known naturally occurring physical systems that behave in this manner. Yet somehow, photosynthesis does.

Under most laboratory circumstances, if you want to make an energy transfer 100% efficient, you have to specially prepare a quantum system in a very particular way. You have to ensure that the incident energy is uniform: where every photon possesses the same energy and wavelength, as well as the same direction and momentum. You have to ensure that there’s an absorptive system that won’t dissipate the incident energy: something like a crystalline lattice where all of the internal components are regularly spaced and ordered. And you need to impose as close to “lossless” conditions as possible, where no energy gets lost due to the internal vibrations or rotations of particles, such as propagating excitations known as phonons.
But in the process of photosynthesis, absolutely zero of these conditions are present. The light that comes in is plain old white sunlight: composed of a wide variety of wavelengths, where no two photons have exactly the same energy and momentum. The absorptive system isn’t ordered in any way, as the distances between the various molecules isn’t fixed in a lattice but rather varies tremendously: on scales of several nanometers between even adjacent molecules. And these molecules are all free to both vibrate and rotate; there are no special conditions that prevent these motions from occurring.
That’s what’s so exciting about this new study, published in early July of 2023 in the Proceedings of the National Academies of Science. What they did was start with one of the simplest known examples of photosynthesis in all of nature: a species of photosynthetic bacteria known as purple bacteria (distinct from the blue-green cyanobacteria), one of the most ancient, simplest, and yet most efficient known examples of an organism that undergoes photosynthesis. (The lack of chlorophyll b helps give this bacteria its purple color.)
The key step that the researchers attempted to isolate and study was after the initial absorption of the photon, but before the last re-emitted photon arrived at the photosynthetic reaction center, as those early and final steps are already well-understood. But in order to understand exactly why this process was so lossless in terms of energy, those intermediate steps need to be quantified and pinned down. That’s also the hard part of this problem, and why it makes so much sense to pick a bacterial system to study that’s so simple, ancient, and yet efficient all at once.
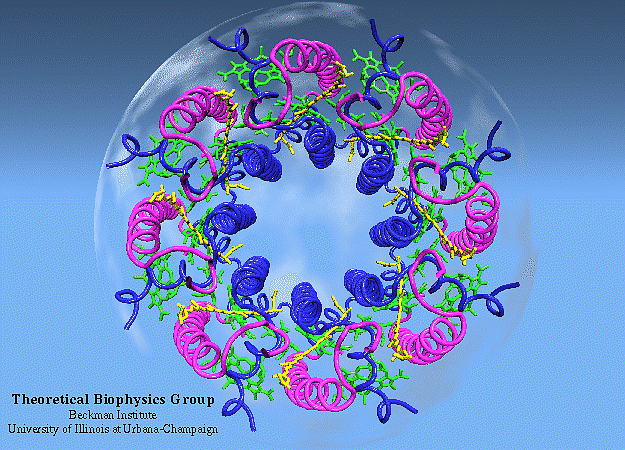
The way the researchers approached the problem was to attempt to quantify and understand how energy was transferred between those series of proteins — known as antenna proteins — to reach the photosynthetic reaction center. It’s important to remember that, unlike in most physical laboratory systems, there isn’t an “organization” to the protein network in biological systems; they’re located and spaced irregularly from one another in what’s known as a heterogeneous fashion, where each protein-protein distance is different from the last.
The primary antenna protein in purple bacteria is known as LH2: for light-harvesting complex 2. Whereas, in purple bacteria, the protein known as LH1 (light-harvesting complex 1) is tightly bound to the photosynthetic reaction center, LH2 is distributed elsewhere, and its biological function is to collect and funnel energy toward the reaction center. In order to perform direct experiments on these LH2 antenna proteins, two separate variants of the protein (conventional LH2 and a low-light variant known as LH3) were embedded into a small-scale disc that is similar to, but slightly different from, the native membrane in which these light-harvesting proteins are naturally found. These near-native membrane discs are known as nanodiscs, and by varying the size of the nanodiscs used in these experiments, the researchers were able to replicate how energy transfer behaved between proteins at a variety of distances.
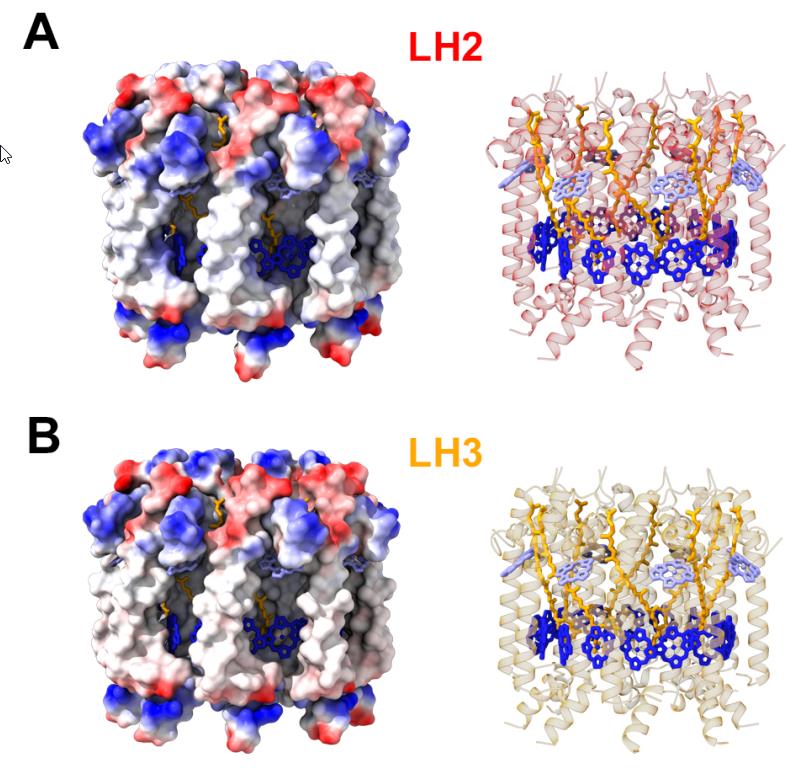
What the researchers found is that as they varied the sizes of the disks, from 25 to 28 to 31 Ångströms, they found that the interprotein energy transfer timescale increased rapidly: from a minimum of 5.7 picoseconds (where a picosecond is a trillionth of a second) to a maximum of 14 picoseconds. When they combined these experimental results with simulations that better represent the actual physical environment found within purple bacteria, they were able to show that the presence of these steps that transfer energy quickly between adjacent antenna proteins can greatly enhance both the efficiency and the distance over which energy can be transported.
In other words, it’s these pairwise interactions between closely-spaced LH2 (and LH3) proteins that likely serves as the key mediator of energy transport: from the moment the first incident photon from sunlight gets absorbed all the way until that energy is finally shepherded into the photosynthetic reaction center. A key finding of this research — a finding that will no doubt be surprising to many — is that these light-harvesting proteins can only very efficiently transfer this energy over long distances because of the irregular and disordered spacing of proteins within the purple bacteria themselves. If the arrangement was regular, periodic, or organized in a conventional way, this long-distance, high-efficiency energy transport could not occur.

And this is what the researchers actually found in their studies. If the proteins were arranged in a periodic lattice structure, the energy transfer was less efficient than if the proteins were arranged in a “randomly organized” pattern, the latter of which is far more representative of how protein arrangements normally occur within living cells. According to the senior author of this latest study, MIT professor Gabriela Schlau-Cohen:
“When a photon gets absorbed, you only have so long before that energy gets lost through unwanted processes such as nonradiative decay, so the faster it can get converted, the more efficient it will be… Ordered organization is actually less efficient than the disordered organization of biology, which we think is really interesting because biology tends to be disordered. This finding tells us that [the disordered nature of systems] may not just be an inevitable downside of biology, but organisms may have evolved to take advantage of it.”
In other words, what we normally consider a “bug” of biology, that biological systems are inherently disordered by many metrics, may actually be the key to how photosynthesis occurs at all in nature.
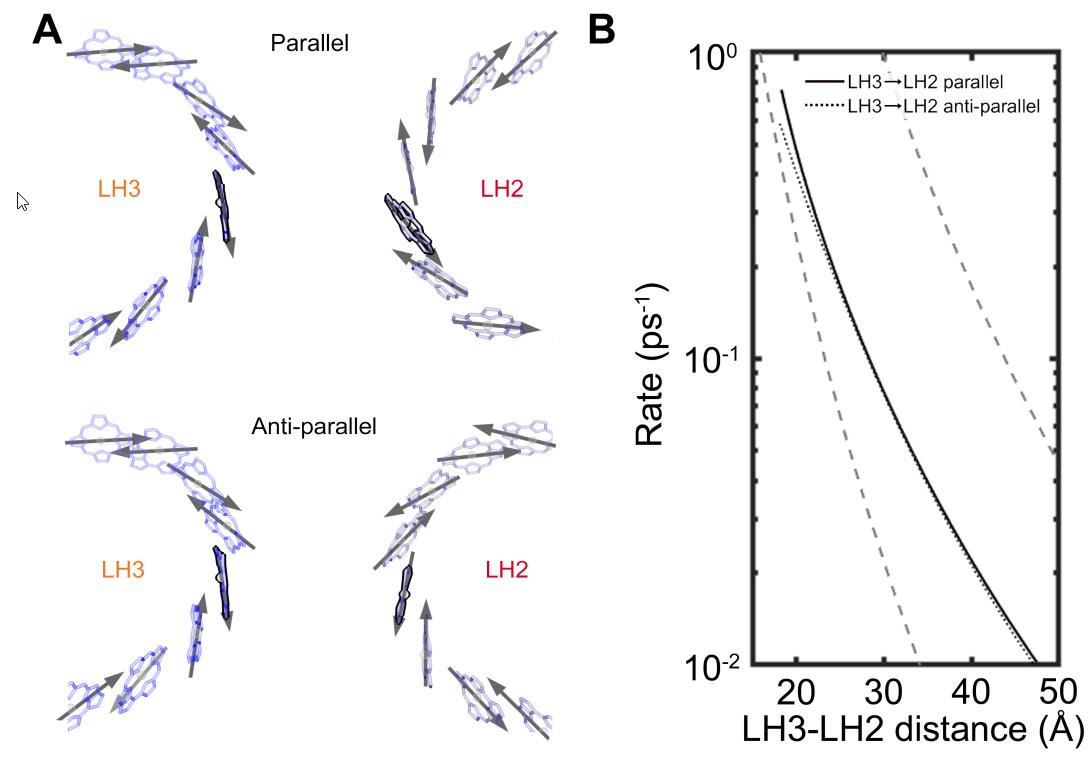
If these antenna proteins had been arranged in a particularly ordered fashion, both in terms of distances from one another and their orientations relative to one another, energy transfer would be slower and more inefficient. Instead, because of the way nature actually works, these proteins are at a variety of irregular distances and at random orientations to one another, enabling for fast, efficient energy transfer toward the photosynthetic reaction center. This key insight, arising from a mix of experiments, theory, and simulations, has finally pointed the way toward a pathway for how this ultra-fast, ultra-efficient energy transfer of sunlight energy occurs, bringing it directly to the photosynthetic reaction center.
We normally think of quantum physics as only being relevant for the simplest of systems: for individual quantum particles or electrons and photons that interact. In truth, however, it’s the underlying explanation behind every non-gravitational phenomenon in our macroscopic world: from how particles bind together to form atoms to how atoms join to make molecules to the chemical reactions that occur between atoms and molecules to how photons are absorbed and emitted by those atoms and molecules. In the process of photosynthesis, by bringing together our combined knowledge of biology, chemistry, and quantum physics, we’re finally solving the mystery of how one of the most energy-efficient processes in all of life science actually occurs.