How Do We Know How Small An Elementary Particle Is?
When we split something into its most fundamental, indivisible components, are we truly seeing something that’s point-like, or is there a finite minimum size?
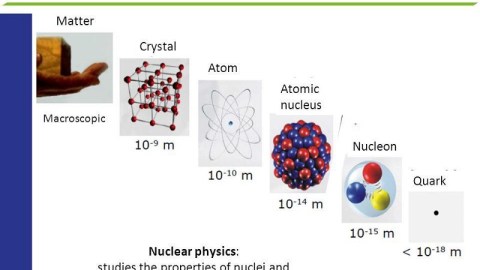
Imagine that you wanted to know what the matter around you was made of, at a fundamental level. You might approach the problem by splitting a piece of that matter into smaller chunks, and then splitting a chunk into tinier pieces, and so on and so on, until you could split it no longer. When you reached your limit, that would be the best approximation of “fundamental” you were able to arrive at.
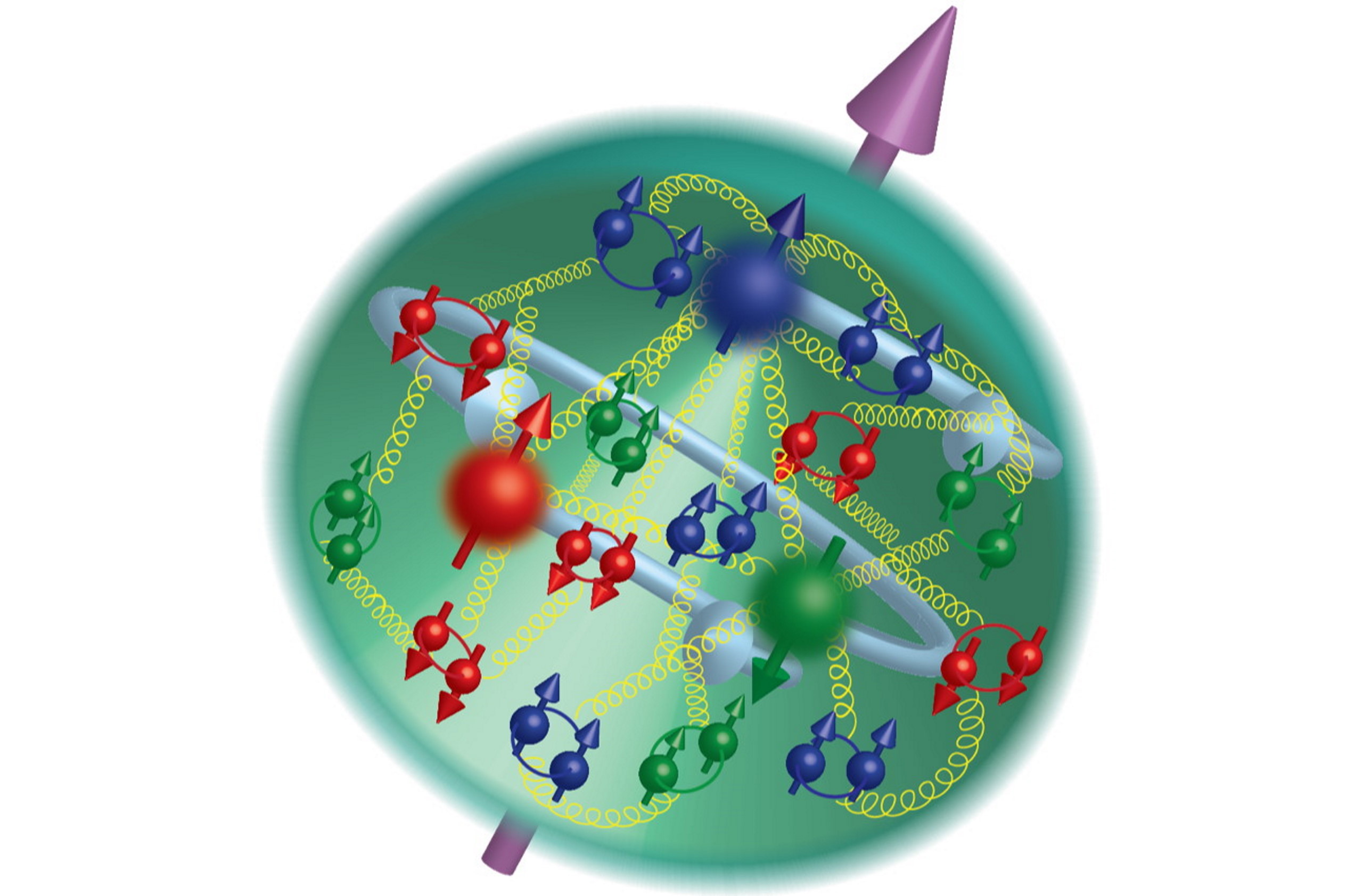
For most of the 19th century, we thought that atoms were fundamental; the Greek word itself, ἄτομος, literally means “uncuttable.” Today, we know that atoms can be split into nuclei and electrons, and that while we cannot split the electron, nuclei can be broken up into protons and neutrons, which can be further subdivided into quarks and gluons. Many of us wonder if they might someday be split further, and how small their size truly is.
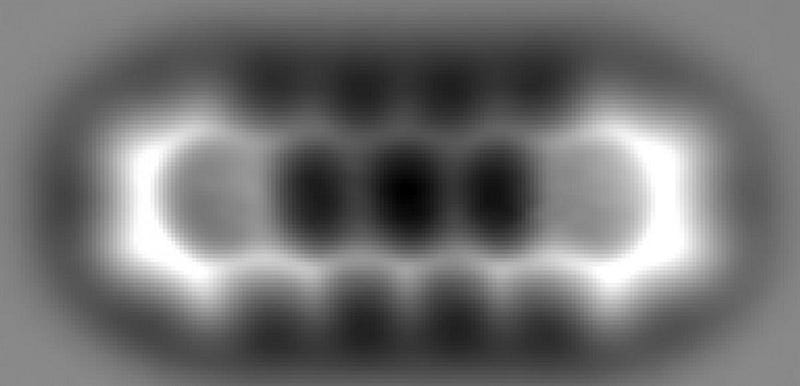
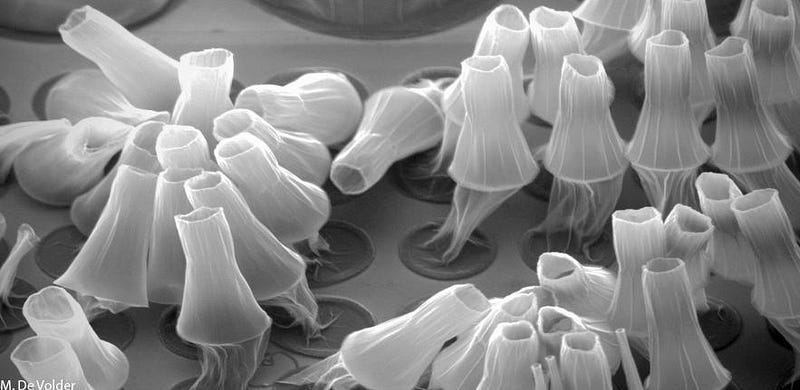
The picture that you see, above, is truly remarkable: it’s an image of individual atoms, arranged in a particular configuration, taken with a technique that’s not so different from an old-style photograph. The way photos work is that light of a particular wavelength or set of wavelengths is sent at an object, some of those light waves travel through unimpeded while others are reflected, and by either measuring the unaffected or the reflected light, you can construct either a negative or positive image of your object.
All of this is dependent on the photographer taking advantage of a particular property of light: the fact that it behaves as a wave. All waves have a wavelength, or a characteristic length scale to them. So long as the object you’re trying to image is larger than the wavelength of the light wave you’re using, you’re going to be able to taken an image of that object.

This gives us a tremendous amount of control over how we choose to look at a particular object: we need to choose an imaging wavelength that will give us high-quality resolution of the object that we want, but that won’t be of such a short wavelength that the act of observing it damages or destroys it. After all, the amount of energy something has increases at shorter and shorter wavelengths.
These choices help explain why:
- we need relatively large antennae to pick up radio waves, because broadcast radio is at a long wavelength and you need a comparably sized antenna to interact with that signal,
- why you have holes in the door of your microwave oven, so that the long-wavelength microwave light gets reflected and stays inside, but the short-wavelength visible light can come out, allowing you to see what’s in there,
- and why the tiny dust grains in space are great at blocking short-wavelength (blue) light, are less good at longer-wavelength (red) light, and are absolutely lousy at blocking even longer-wavelength (infrared) light.

You might assume that photons, or quanta of light, are really the way to go when it comes to imaging objects on all scales. After all, if you want to construct an image of something, why wouldn’t you use light?
The thing is, physics doesn’t care whether you’re a photon or not in constructing an image. All physics cares about is what your wavelength is. If you’re a quantum of light, that’s going to be your photon wavelength. But if you’re a different quantum particle, like an electron, you’re still going to have a wavelength that’s related to your energy: your de Broglie wavelength. In reality, whether you choose to use a light wave or a matter wave is irrelevant. All that matters is the wavelength. That’s how we can probe matter, and determine the size of an object, down to any arbitrary scale we choose.
This property of matter was such a surprise when it was first revealed that scientists studied it ad nauseum, baffled and shocked at what they saw. If you fired an electron through a slit in a barrier, it would show up in a small pile on the other side. If you cut a second slit very close to the first one, though, you wouldn’t get two piles; instead, you’d get an interference pattern. It was as though your electrons were truly behaving as waves.
Things got even weirder when people tried to control the electrons, firing them one-at-a-time towards these two slits. They set up experiments to record where the electrons landed, one-at-a-time, on a screen behind the slit. As you fired more electrons, one after the other, that same interference pattern began to emerge. Not only did electrons behave as waves, but each one acted as though it could interfere with itself.
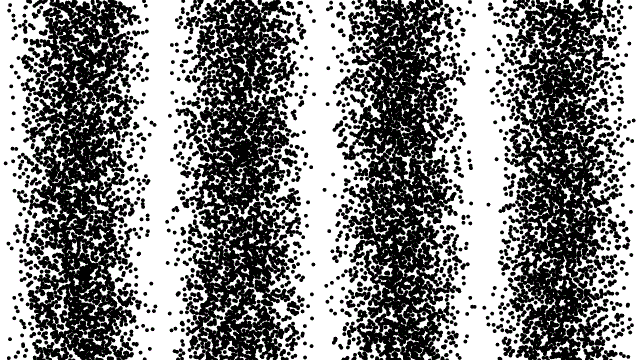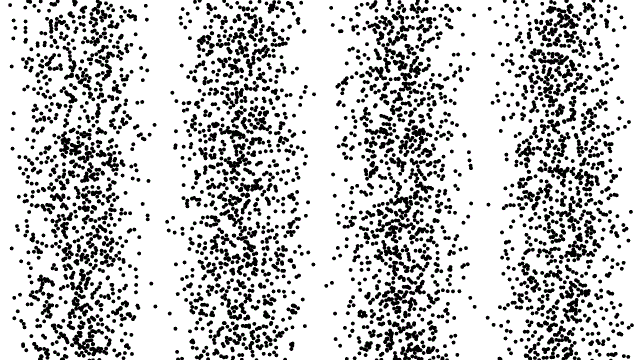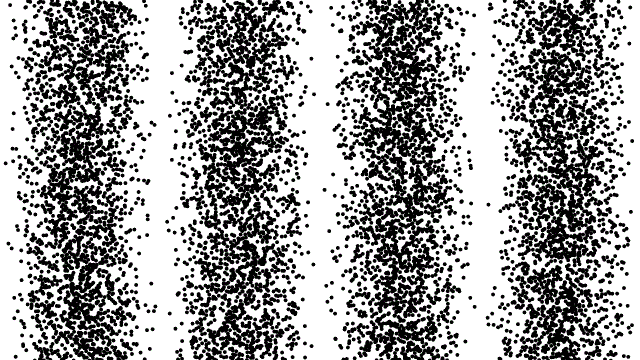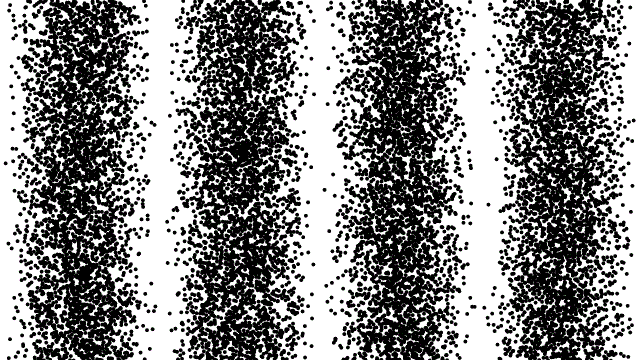
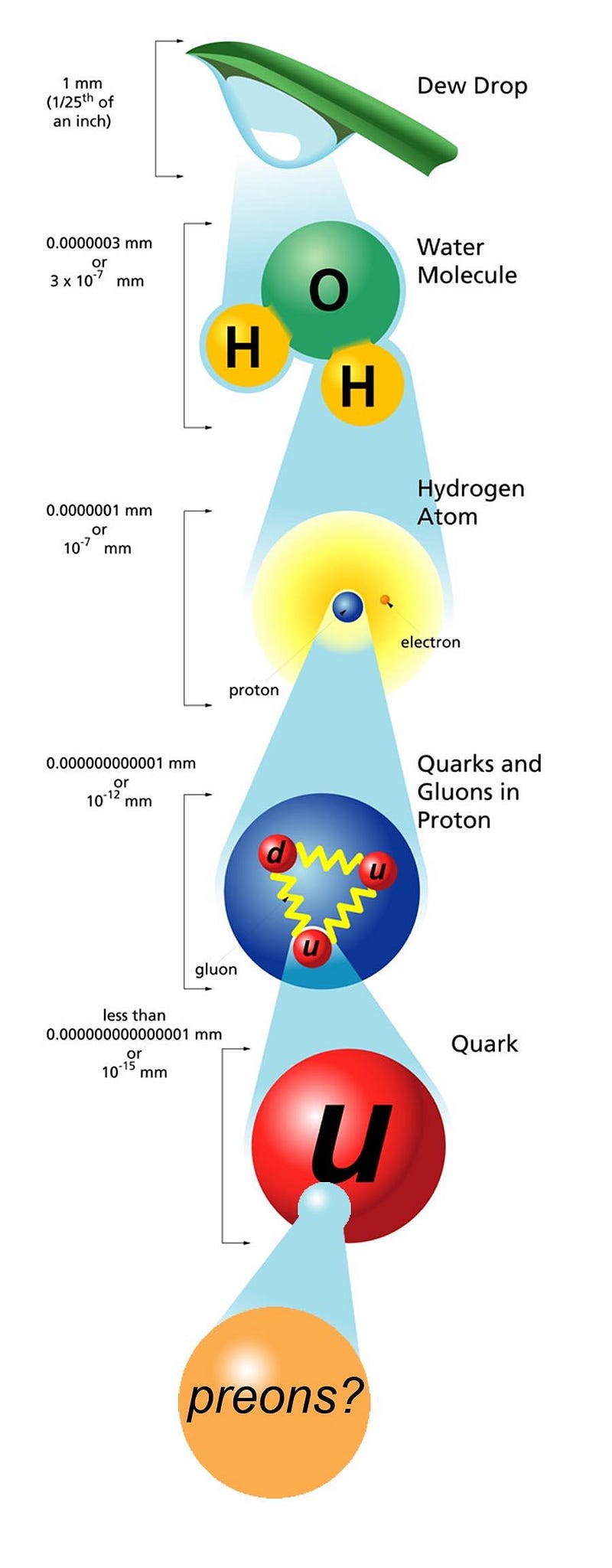
The higher-energy you can cause your particle to achieve, the smaller the size of a structure you can probe. If you can crank up the energy on your electrons (or photons, or protons, or what have you), the shorter your wavelength and the better your resolution. If you can measure exactly when your non-fundamental particle splits apart, you can determine that energy threshold and, therefore, its size.
This technique enabled us to determine that:
- Atoms aren’t indivisible, but are made of electrons and nuclei with a combined size of ~1 Å, or 10^-10 meters.
- Atomic nuclei can be split apart into protons and neutrons, each with a size of ~1 fm, or 10^-15 meters.
- And if you bombard electrons or quarks or gluons with high-energy particles, they show no evidence of internal structure, down to a size of ~10^-19 meters.
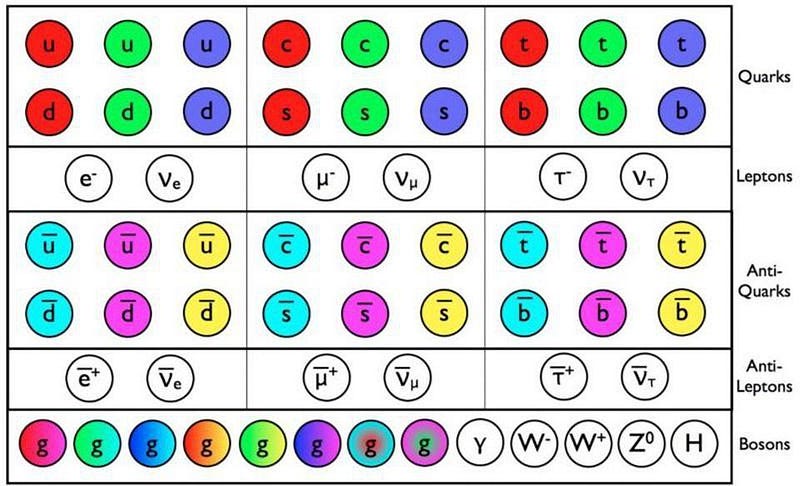
Today, we believe, based on our measurements, that each of the Standard Model particles is fundamental, at least down to this scale of 10^-19 meters.
Fundamental, we believe, should mean that the particle is absolutely indivisible: it cannot be broken apart into smaller entities that make it up. In simpler terms, we should not be able to crack it open. According to our best theory of particle physics, the Standard Model, all of the known particles:
- the six types of quarks and six antiquarks,
- the three charged leptons and three antileptons,
- the three neutrinos and antineutrinos,
- the eight gluons,
- the photon,
- the W and Z bosons,
- and the Higgs boson,
are expected to be indivisible and fundamental and point-like.
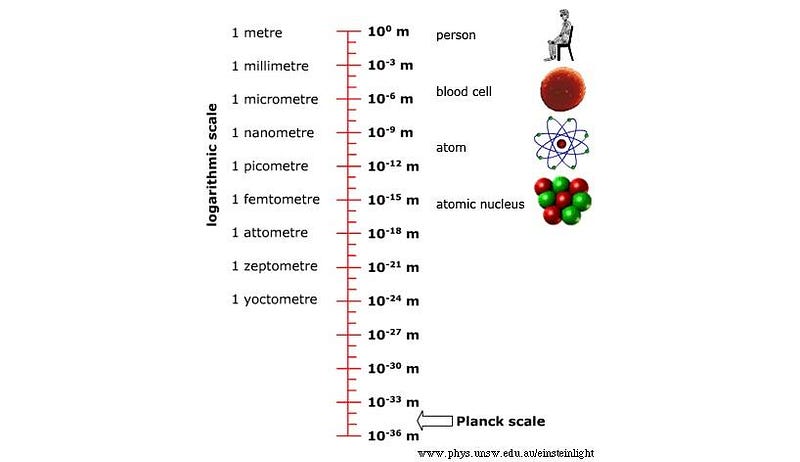
But here’s the thing: we don’t know that this is true. Sure, the Standard Model says that this is the way that things are, but we know that the Standard Model doesn’t give us the final answer to everything. In fact, we know that at some level, the Standard Model must break down and be wrong, because it doesn’t account for gravity, dark matter, dark energy, or the preponderance of matter (and not antimatter) in the Universe.
There has to be something out there more to nature than this. And maybe it’s because the particles that we think are fundamental, point-like, and indivisible today actually aren’t. Perhaps, if we go to high-enough energies and small-enough wavelengths, we’ll be able to see that at some point, between our current energy scales and the Planck energy scale, there’s actually more to the Universe than we presently know.
When it comes to the fundamental particles of nature, this technique of smashing particles into one another is the best tool we have to investigate them. The fact that none of these fundamental particles have cracked apart, shown an interior structure, or given us a hint that they have a finite size to them are the best evidence we have, to date, concerning their nature.
But the curious among us won’t simply be satisfied with the present limits that we’ve set. If we had stopped at atoms, we never would have discovered the quantum secrets that lie within the atom. If we had stopped with protons and neutrons, we never would have discovered the underlying structure of the normal matter that fills the Universe. And if we stop here, with the Standard Model, who knows what we’ll be missing?
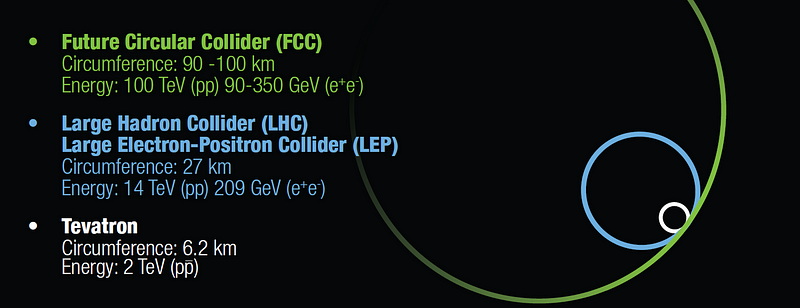
Science is not some half-baked enterprise, where we know the answers going into an experiment and only perform it to confirm what we know. Science is about discovery. It’s about looking where we’ve never looked before, and finding out what lies behind that veil of uncertainty. The day may come where all of humanity takes a look at what we know and the magnitude of what we’d have to build to take that next step and say, “there’s no way we can do that,” but that’s not where we are today.
We know how to go to the next level. We know how to go to the next order-of-magnitude and the next significant digit in energy and in size. Is the Universe we understand today truly all there is out there? It can’t be. Until we’ve discovered the last of nature’s secrets about what’s truly fundamental, we cannot allow ourselves to stop the search.
Ethan Siegel is the author of Beyond the Galaxy and Treknology. You can pre-order his third book, currently in development: the Encyclopaedia Cosmologica.