Aliens? Or Alien Impostors? Finding Oxygen Might Not Mean Life, After All

The most surefire, easily-seen signature of life on Earth might be a cosmic red herring around other worlds.
In our quest for life beyond the Solar System, it makes sense to look for a world like our own. We’ve long hoped to find an Earth-sized world around a Sun-like star at the right distance for liquid water as our first step, and with thousands of planets in our coffers already, we’re extremely close. But not every world with the right physical properties is going to have life; we need additional information to know whether a potentially habitable world is actually inhabited.
The follow-up would be to analyze the planet’s atmosphere for Earth-like signatures: potential signs of life. Earth’s combination of atmospheric gases — nitrogen, oxygen, water vapor, carbon dioxide and more — has been assumed to be a dead giveaway for a planet with life on it. But a new study by planetary scientist Dr. Sarah Hörst’s team throws that into doubt. Even worlds rich in oxygen might not harbor aliens, but an impostor process that could fool us all.
The scientific story of how to even reach that point is fascinating, and closer to becoming a reality than ever before. We can understand how this happens by imagining we were aliens, looking at our Sun from a large distance away, trying to determine if it possessed an inhabited world.
By measuring the slight variations in the frequency of the Sun’s light over long periods of time, we’d be able to deduce the gravitational influence of the planets on them. This detection method is known either the radial velocity or the stellar wobble method, and can tell us information about a planet’s mass and orbital period. Most of the early (pre-Kepler) exoplanets were discovered with this technique, and it’s still the best method we have for both determining planetary masses and confirming the existence of candidate exoplanets.
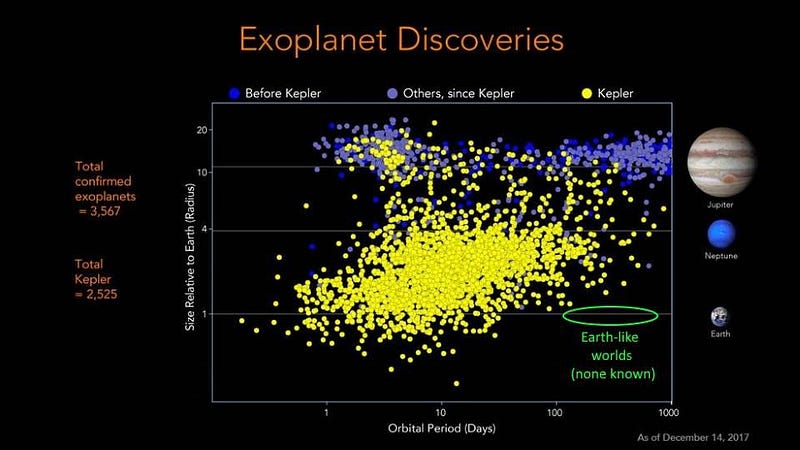
We also need to know the size of the planet. With the stellar wobble alone, we’ll only know what the mass of the world is relative to the angle-of-inclination of its orbit. A world that’s the mass of Earth could be well-suited to life if it’s got an Earth-like atmosphere, but it could be disastrous for life if it’s an iron-like world with no atmosphere at all, or a low-density, puffy world with a large gaseous envelope.
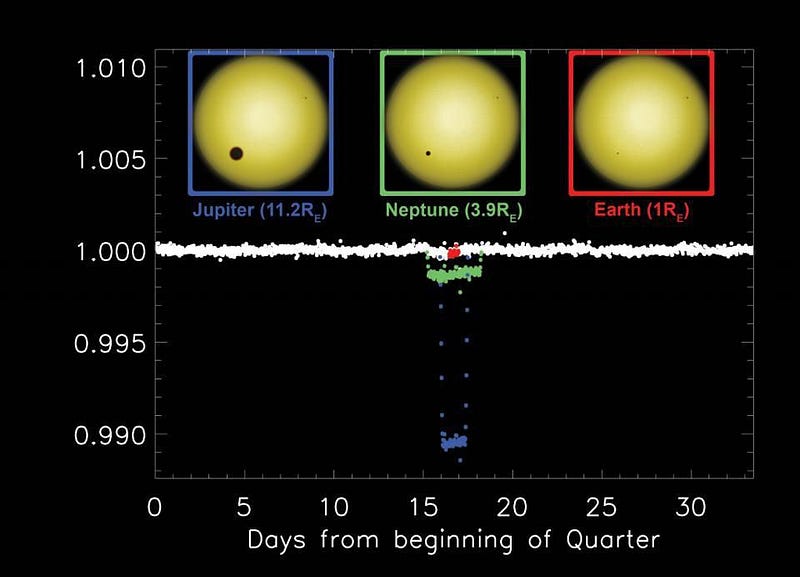
The transit method, where a planet passes in front of its parent star, is our most prolific method for measuring a planet’s radius. By calculating how much of the parent star’s light it blocks when it crosses our line-of-sight, we can determine its size. For an alien civilization whose line-of-sight was properly aligned with Earth orbiting the Sun, we’d be able to detect it with technology only about 20% more sensitive than Kepler was.
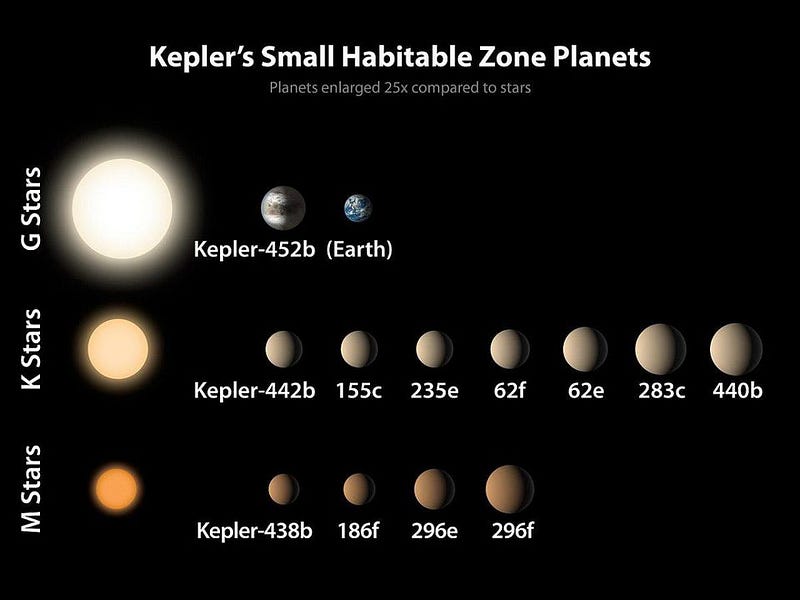
This is roughly where we are today. We’ve found hundreds of worlds that we suspect are rocky orbiting their stars, many of them right around Earth-sized. For a large fraction of them, we’ve measured their mass, radius, and orbital period, with a small percentage being at the right orbital distance to have Earth-like temperatures.
Most of them orbit red dwarf stars — the most common class of star in the Universe — which means the forces should tidally lock them: the same side should always face the star. These stars flare often, posing a danger to any potential atmospheres on these worlds.
But a significant fraction will orbit K, G, or F-class stars, where they can rotate on their axes, maintain an atmosphere, and have the potential for Earth-like life. That’s where we want to look.
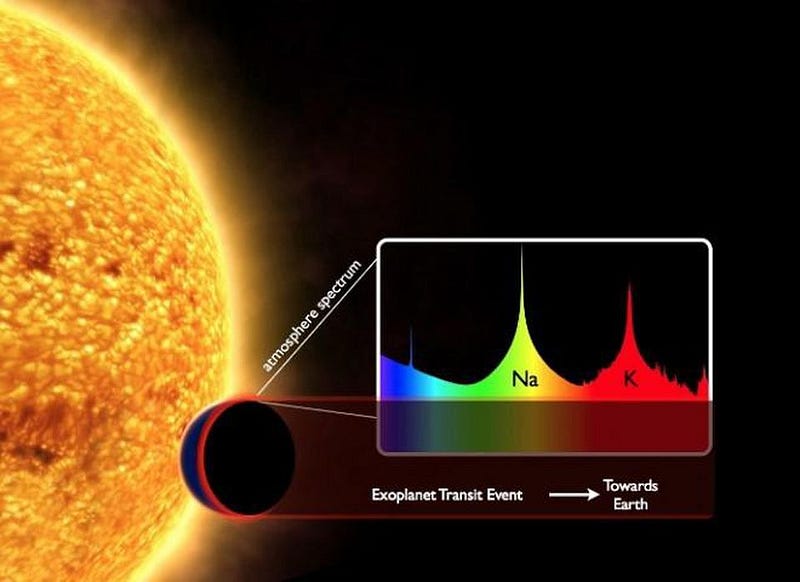
And that’s where future technology is hoping to take us. If a larger Kepler-like telescope were equipped with the right instruments, we could break up the light passing through an exoplanet’s atmosphere during a transit, and determine its atomic and molecular contents. If we were looking at Earth, we could determine that it was composed of nitrogen, oxygen, argon, water vapor, and carbon dioxide, along with other trace signatures.
Even without an ideal alignment, direct imaging will still be possible. Potential NASA flagship missions, such as HabEx or LUVOIR (with either a starshade or a coronagraph), could block the light of the parent star and detect the light from an orbiting planet directly. This light could again be broken up into its individual wavelengths, determining its molecular content.
Whether from absorption (transit) or emission (direct imaging), we could learn what a potential Earth-twin’s atmosphere is composed of.
So what if we find an oxygen-rich world? No other planets, dwarf planets, moons, or other objects contain even 1% oxygen that we know of. Earth’s atmosphere transformed over nearly 2 billion years before it had an oxygen content comparable to what it does today, and it was anaerobic life processes that created our modern atmosphere that’s rich in molecular oxygen. Because of how easily oxygen is destroyed by ultraviolet light and how difficult it is to produce in large quantities via inorganic, chemical processes, oxygen has long been taken as the one biosignature we could rely on to indicate a living world.
If organic molecules were found there as well, it would seem like a surefire indicator that life, indeed, must have taken hold on such a planet.

And that’s where the Hörst lab’s new findings come into play. In a paper just published in ACS Earth and Space Chemistry, a specially-designed chamber to mimic the environment of a hazy exoplanet atmosphere showed that molecular oxygen (O2) could be created in a number of environmental conditions likely to occur naturally, with no life necessary to create it.
The ingenious method was to create a gas mixture that would be consistent with what we expect an Earth-like or super-Earth-like environment might hold. That mixture was then inserted into a specially-designed chamber and subjected to a variety of temperature, pressure, and energy-injection conditions that would likely mimic the activity that could occur on actual exoplanets.

A total of nine different gas mixtures were used at temperatures ranging from 27 °C (80 °F) up to approximately 370 °C (700 °F), representing the temperature range expected to naturally occur. The energy injection came in two different forms: from ultraviolet light and from plasma discharges, which represent natural conditions likely to be caused by sunlight or lightning-like activity.
The results? There were multiple scenarios that resulted in the production of both organic molecules (like sugar and amino acid precursors) and oxygen, yet didn’t require any life at all to get them. According to first author Chao He,
People used to suggest that oxygen and organics being present together indicates life, but we produced them abiotically in multiple simulations. This suggests that even the co-presence of commonly accepted biosignatures could be a false positive for life.
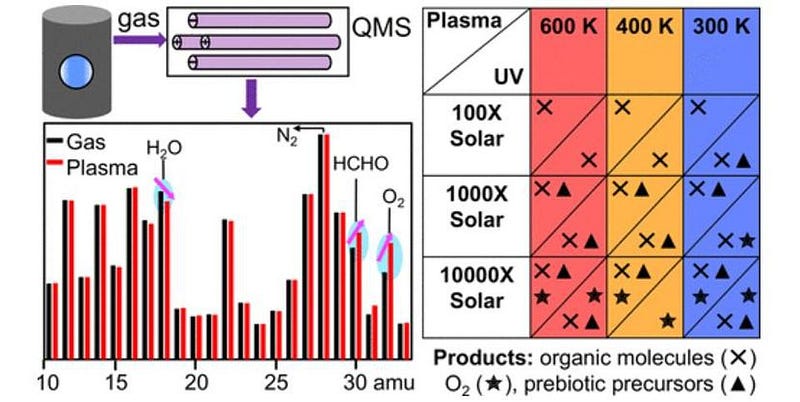
The experiment wasn’t some cherry-picked design to attempt to produce this false-positive result, either. The gases inside the chamber were designed to mimic the contents of known exoplanetary atmospheres, with the ultraviolet energy injection designed to simulate sunlight. The experiments simulated a variety of atmospheric (hydrogen-rich, water-rich, and carbon dioxide-rich) environments, and all of them created haze particles and yielded organic molecules such as hydrogen cyanide, acetylene, and methanimine.

Multiple environments generated organic molecules, prebiotic precursor molecules, and oxygen all at once, at Earth-like temperatures and much hotter temperatures as well. The paper itself states the main conclusion very succinctly:
Our laboratory results indicate that complex atmospheric photochemistry can happen in diverse exoplanet atmospheres and lead to the formation of new gas products and haze particles, including compounds (O2 and organics) that could be falsely identified as biosignatures.
The amount of molecular oxygen produced in these experiments was relatively small by some metrics; Hörst herself wouldn’t call the atmospheres created in the lab “oxygen-rich.” But it’s nevertheless possible that these processes would translate into an oxygen-rich atmosphere on an exoplanet, given the right conditions and enough time. At this point, it appears possible that finding the presence of both organics and molecular oxygen could be due to abiotic, non-life processes exclusively.
This doesn’t mean that finding an Earth-like world with an oxygen-rich atmosphere won’t be incredibly interesting; it absolutely will be. It doesn’t mean that finding organic molecules coincident with the oxygen won’t be compelling; it will be a finding worth getting excited over. It doesn’t even mean that it won’t be indicative of life; a world with oxygen and organic molecules may well be overflowing with living organisms. But it does mean that we have to be careful.
Historically, when we’ve looked to the skies for evidence of life beyond Earth, we’ve been biased by hope and what we know on Earth. Theories of dinosaurs on Venus or canals on Mars still linger in our memories, and we must be careful that extraterrestial oxygen signatures don’t lead us to falsely optimistic conclusions. We now know that both abiotic processes and life-dependent ones can create an oxygen-rich atmosphere.
The hard problem, then, will be disentangling the potential causes when we actually find our first oxygen-rich, Earth-like exoplanet. Our reward, if we’re successful, will be the knowledge of whether or not we’ve actually found life around another star.
Ethan Siegel is the author of Beyond the Galaxy and Treknology. You can pre-order his third book, currently in development: the Encyclopaedia Cosmologica.





