Researchers discover an entirely new way to treat anxiety

- Roughly a third of all Americans will experience an anxiety disorder at some point in their lives.
- While leading anti-anxiety medications are generally effective and well-tolerated, they come with problematic drawbacks.
- In a new study, researchers discovered a signaling pathway that when slowed down via a therapeutic injection, effectively halted anxious behaviors in mice.
Researchers at the National Institute of Neurological Disorders and Stroke (NINDS) have discovered a new way to treat anxiety. By manipulating a specific signaling pathway in the brains of young, stressed mice, they effectively halted the animals’ anxious behaviors. The advance could one day lead to novel anti-anxiety medications that target the pathway in humans.
NINDS research fellow Saurabh Pandey and senior investigator Wei Lu spearheaded the work. They detailed their efforts in a paper published in the Proceedings of the National Academy of Sciences.
Roughly a third of all Americans experience an anxiety disorder at some point in their lives. These ailments include general anxiety disorder, severe phobias, panic disorder, social anxiety disorder, and post-traumatic stress disorder. They’re often treated with mental health therapy or medications.
For instance, benzodiazepines are a class of anti-anxiety drugs that are generally effective and well-tolerated; however, they come with problematic drawbacks. They can be addictive, interact dangerously with certain other drugs, and often trigger wide-ranging side effects such as drowsiness, confusion, headache, nausea, and tremors.
These drugs work by enhancing the activity of receptors that respond to an inhibitory neurotransmitter called gamma-aminobutyric acid (GABA). GABA reduces the excitability of neurons throughout the nervous system, essentially slowing down most bodily processes. This global deceleration also triggers the benzodiazepines’ calming effect. Familiar brand names of the drug include Valium, Xanax, Ativan, and Klonopin.
The NINDS team wondered whether there was a better, more precise way to treat anxiety — one that wouldn’t trigger body-wide side effects. So, they stressed out mice until the animals developed clear signs of chronic anxiety when compared to their healthy, unstressed companions. For example, they were less willing to explore new areas, engaged in more repetitive behaviors, and avoided social interactions.
It was almost as if the researchers found an off-switch for anxiety in the mouse brain.
The researchers then searched for differences between the brains of anxious and unstressed mice. They noticed that levels of two neurotransmitter proteins, Neuroligin2 and GABA, were markedly decreased in the anxious mice. Already aware of GABA’s ability to lessen anxiety, they focused on Neuroligin2. With further probing, the researchers determined that chronic stress enhances the activity of a protein called Src kinase, triggering a cascade of effects called a signaling pathway that results in less circulating Neuroligin2. They wondered: Would suppressing Src kinase’s activity help alleviate the rodents’ anxiety?
To find out, Pandey, Lu, and their colleagues injected the anxious mice with an Src-inhibiting therapeutic called PP2 once daily for seven days. The effects were astounding. With less Src activity, its corresponding signaling pathway slowed to a standstill, resulting in higher Neuroligin2. Outwardly, the animals’ anxious behaviors disappeared. Moreover, there were no noticeable adverse effects. It was almost as if the researchers found an off-switch for anxiety in the mouse brain.
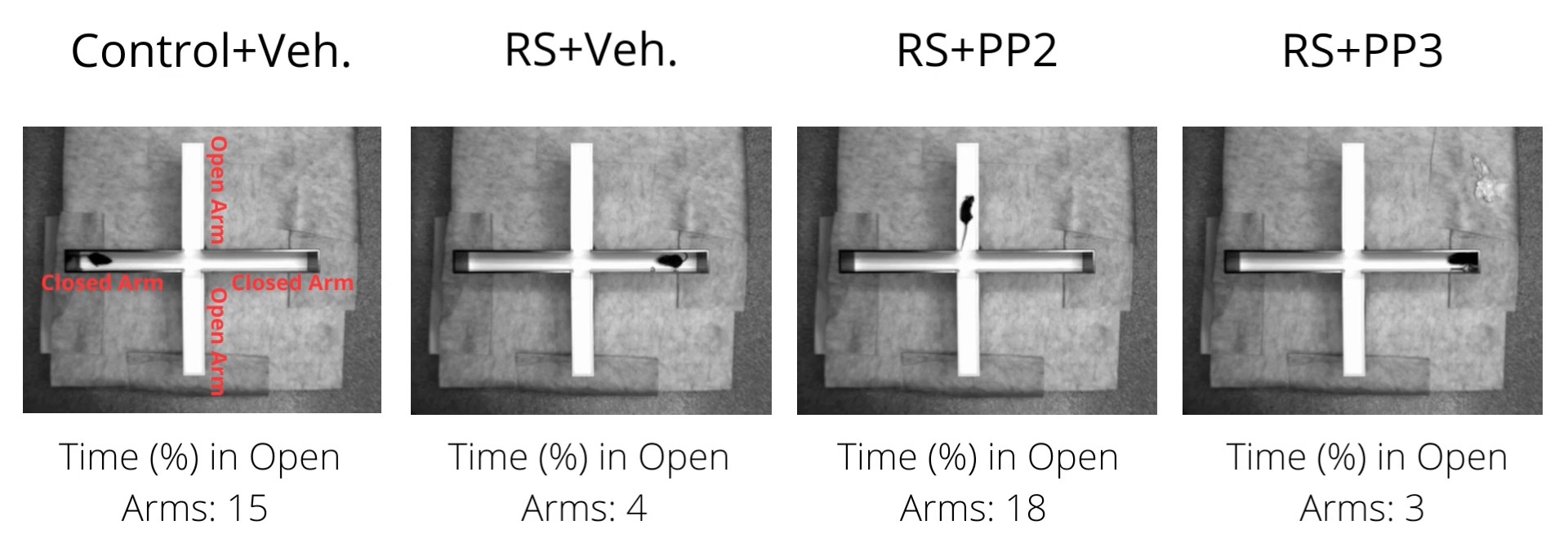
“The reversal of elevated anxiety-like behaviors […] suggests a powerful mechanism for potential therapeutic applications,” the researchers commented in their paper.
As fellow mammals, humans also have this signaling pathway, potentially opening the door to a brand new class of medications to treat anxiety. Such drugs would arrive years down the road, of course, following further animal testing and clinical trials.
“We would love to extend this study further to the pre-clinical and clinical stage,” Lu tells Big Think. “If we get the opportunity to collaborate with the relevant experts in the industry, this will definitely be a future direction.”
“In principle, PP2 can be adopted in the pill form; however, metabolic and dosage standardization would be the next logical step,” he adds.
The NINDS researchers aren’t the first team to search for ways to effectively switch off anxiety in the brain. Last year, scientists primarily based out of the University of Exeter in the U.K. identified a gene called Pgap2 in the mouse brain’s amygdala that, when suppressed, reduced the animals’ anxiety symptoms.
As a whole, the physical causes of anxiety disorders in the brain remain poorly understood. Elucidating them with state-of-the-art techniques might one day allow us to essentially program problematic anxiety out of the brain.