How some plants became carnivorous predators

Toward the end of the 19th century, lurid tales of killer plants began popping up everywhere. Terrible, tentacle-waving trees snatched and swallowed unwary travelers in far-off lands. Mad professors raised monstrous sundews and pitcher plants on raw steak until their ravenous creations turned and ate them too.
The young Arthur Conan Doyle stuck closer to the science in a yarn featuring everyone’s favorite flesh-eater, the Venus flytrap. Drawing on brand-new botanical revelations, he accurately described the two-lobed traps, the way they captured insects, and how thoroughly they digested their prey. But even his flytraps were improbably large, big enough to entomb and consume a human. Meat-eating, man-eating plants were having a moment, and for that you can thank Charles Darwin.
Until Darwin’s day, most people refused to believe that plants ate animals. It was against the natural order of things. Mobile animals did the eating; plants were food and couldn’t move — if they killed, it must only be in self-defense or by accident. Darwin spent 16 years performing meticulous experiments that proved otherwise. He showed that the leaves of some plants had been transformed into ingenious structures that not only trapped insects and other small creatures but also digested them and absorbed the nutrients released from their corpses.
In 1875, Darwin published Insectivorous Plants, detailing all he had discovered. In 1880, he published another myth-busting book, The Power of Movement in Plants. The realization that plants could move as well as kill inspired not just a hugely popular genre of horror stories but also generations of biologists eager to understand plants with such unlikely habits.
Today, carnivorous plants are having another big moment as researchers begin to get answers to one of botany’s great unsolved riddles: How did typically mild-mannered flowering plants evolve into murderous meat-eaters?

Since Darwin’s discoveries, botanists, ecologists, entomologists, physiologists and molecular biologists have explored every aspect of these plants that drown prey in fluid-filled pitchers, immobilize them with adhesive “flypaper” leaves or imprison them in snap-traps and underwater suction traps. They’ve detailed what the plants catch and how — plus something of the benefits and costs of their quirky lifestyle.
More recently, advances in molecular science have helped researchers understand key mechanisms underpinning the carnivorous lifestyle: how a flytrap snaps so fast, for instance, and how it morphs into an insect-juicing “stomach” and then into an “intestine” to absorb the remains of its prey. But the big question remained: How did evolution equip these dietary mavericks with the means to eat meat?
Fossils have provided almost no clues. There are very few, and fossils can’t show molecular details that might hint at an explanation, says biophysicist Rainer Hedrich of the University of Würzburg in Germany, who explores the origins of carnivory in the 2021 Annual Review of Plant Biology. Innovations in DNA sequencing technology now mean that researchers can tackle the question another way, searching for genes linked to carnivory, pinpointing when and where those genes are switched on, and tracing their origins.
There’s no evidence that carnivorous plants acquired any of their beastly habits by hijacking genes from their animal victims, says Hedrich, although genes do sometimes pass from one type of organism to another. Instead, a slew of recent findings point to the co-option and repurposing of existing genes that have age-old functions ubiquitous among flowering plants.
“Evolution is sneaky and flexible. It takes advantage of preexisting tools,” says Victor Albert, a plant-genome biologist at the University at Buffalo. “It’s simpler in evolution to repurpose something than make something new.”
Road to predation
Quirky though it is, carnivory has evolved repeatedly over the 140 million-plus years that flowering plants have been around. The adaptation arose independently at least 12 times, says Tanya Renner, an evolutionary biologist at Penn State.
Each time, the driving force for evolution was the same: the need to find an alternative source of vital nutrients. Carnivorous plants grow in swamps and bogs, in nutrient-poor bodies of water or on thin tropical soils, all habitats short on the nitrogen and phosphorus essential for growth. Protein-packed insects and other small invertebrates are rich sources of both, as well as other elements plants need to flourish. “A Venus flytrap can live for three weeks on a single large insect,” says Hedrich. “If it captures lots of insects, it produces more leaves and more traps.”
Today there are some 800 known carnivorous species. Some, like pitcher plants and many sundews, are passive receivers of prey — albeit with ingenious adaptations such as slippery rims and gluey-tipped hairs that help to secure a meal. Others are more active: Some sundews curl inward, nudging prey into the trap’s stickier center, while a few have an outer ring of fast-moving tentacles that hurl victims to their doom. Most sophisticated of all is the Venus flytrap, Dionaea muscipula, with its sensitive trigger hairs and snap-traps that can distinguish the touch of an insect from a falling raindrop or dead leaf and can judge the size of prey and respond accordingly.
Despite huge differences in shape and form and mode of killing, all traps are modified leaves or parts of leaves. “That means these plants not only get nutrients from a different source but by a different route, primarily through their leaves rather than their roots,” says Renner.
How did leaves come to perform such un-leaf-like functions? To find out, researchers have turned to a mix of “omics” techniques — genomics, transcriptomics and proteomics. They compare genomes of carnivorous and non-carnivorous plants; sequence the RNA transcripts that carry a gene’s instructions to see which genes are switched on where and when; and draw up inventories of proteins to find out which ones the traps manufacture at mealtimes.
New jobs for old genes
Many features of the carnivorous lifestyle have yet to give up their genetic secrets. But studies of two of its grislier elements — digestion and absorption — are revealing how evolution repurposed existing genes, putting some to work in new places and giving others new functions and the odd tweak to suit them better to their new roles. In many cases, plants that evolved carnivory entirely independently have repurposed the same genes. Faced with the problem of consuming flesh, they all hit on the same solution, Albert says. And central to the transformation was the plant’s age-old system of defense.
Back in the 1970s, researchers recognized that the digestive fluid they found in traps contained enzymes that functioned in very similar ways to many of the chemical weapons that plants wield against harmful bacteria, fungi and hungry herbivorous insects. Initially, it wasn’t clear whether carnivorous plants made the enzymes themselves or if microbes living in their traps did. Since then, botanists have confirmed that carnivorous plants do produce many of those enzymes and have discovered dozens more. Today’s fast and cheap sequencing technology has enabled molecular scientists to identify many of the genes encoding these digestive enzymes and to monitor their activity as plants trap and process prey.
The roster of enzymes includes chitinases, which break down the chitin of insect exoskeletons; flesh-dissolving proteases, which break down proteins; and purple acid phosphatase, which enables plants to extract usable phosphorus from their victims’ deconstructed corpses. All played roles in the ubiquitous and ancient defenses of flowering plants. “The genes for those enzymes were repurposed when plants started to eat the things they were originally protecting themselves from,” says Albert. “Chitinases most likely were for defense against fungi, which have chitin in their cell walls. Later, after arthropods evolved, they helped defend against them.” Protein-digesting enzymes also helped to repulse attackers.
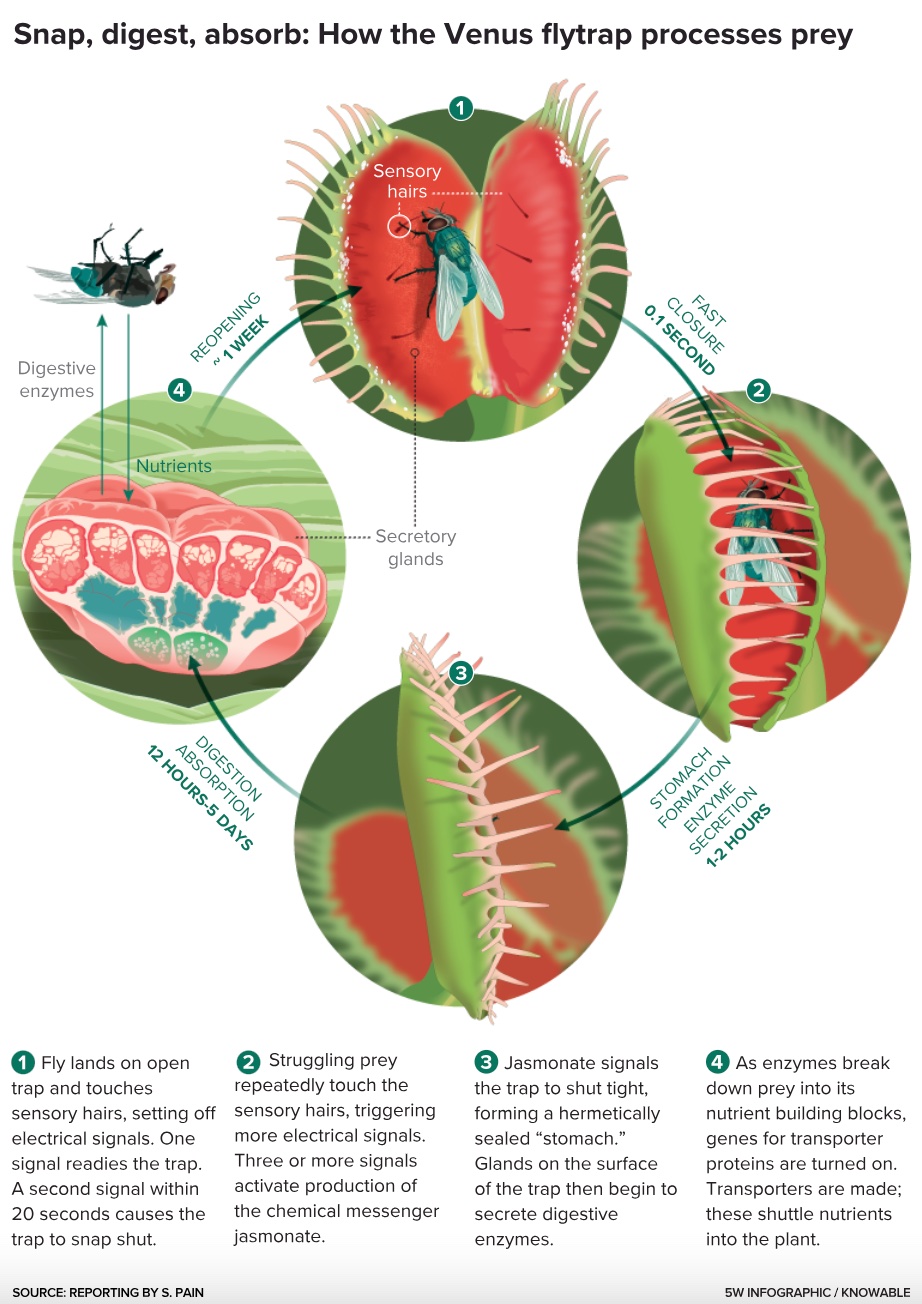
Evolution’s tendency to adopt and adapt existing tools goes beyond digestion. As chitin, proteins and DNA are broken into smaller molecules, the trap must move them from the outside world to the inside of the plant. In ordinary plants, uptake of nutrients is the job of a root, where transporter proteins continually shuttle them from the soil into the plant. “You might not expect to find those proteins working in a leaf,” says Renner.
Yet that’s precisely what Hedrich’s colleague Sönke Scherzer found in the Venus flytrap as it processes prey: He recently identified transporters for two of the most vital plant nutrients, nitrogen and potassium. To enable a leaf to absorb nutrients, it seems, evolution co-opted root genes and put them to work somewhere new. The difference is that transporter genes are always active in roots, but in traps they are switched on only once nutrients begin to flow from decomposing prey.
The way of all flesh-eaters?
Co-option is an important driver of evolutionary innovation, and often begins with the accidental duplication of genes during cell division. Most duplicate genes serve no purpose and are eventually lost. But if spare genes acquire useful mutations, that can pave the way for a change in function. “Duplication of genes is always happening and sometimes it’s highly adaptive,” says Albert. This seems to have been the way carnivorous plants evolved their meat-eating abilities — at least for those genes examined so far.
What came as more of a surprise was the discovery that whenever and wherever a new line of carnivores arose, evolution worked on the same genes.
In 2017, evolutionary biologist Kenji Fukushima, Hedrich’s colleague and coauthor of the 2021 Annual Review article, joined Albert and an international team of researchers to sequence the genome of an Australian carnivorous plant called Cephalotus follicularis. Like many carnivores, it traps prey in pitchers — in this case, small, squat, toothy-mouthed pitchers — but it sits on its own separate branch of the plant family tree.

The team identified many genes linked to different aspects of its meat-eating habits, from how the plant attracts prey to how it makes the inside of its pitchers too slippery for insects to escape. The big surprise came when they probed the origins of digestive enzymes in Cephalotus and three more, unrelated, species: Nepenthes alata (an Asian pitcher plant), the North American pitcher Sarracenia purpurea and a sundew, Drosera adelae. All of them, it turned out, had repurposed the same ancient enzymes — matching ones identified previously in the Venus flytrap. Between them, these five species represent three independent lines of carnivores. This was a classic case of convergent evolution, says Albert. It suggested there were only limited pathways to becoming a carnivorous plant.
Delving deeper, Fukushima discovered that convergent evolution went beyond co-opting the same genes. Once enzymes had taken on their new, carnivory-related roles, they continued to evolve, swapping some of their amino acids for others that improved their performance, probably by prolonging their activity in an inhospitable stew of protein-busting chemicals. Fukushima found the very same amino acid substitutions in unrelated plants.
Fly in the ointment
As they continue to explore carnivory, researchers are identifying many more enzymes. “But time and time again we’re finding that they have similar functions across distantly related species,” says Renner, who heads a major investigation into the role of co-option in the making of meat-eaters. Yet while that bolsters the idea that carnivorous plants acquired their new digestive skills in much the same way, there’s growing suspicion that the same might not be true for the all-important mechanism that controls the whole operation by switching on the right genes at the right time.
The chain of events in trapping and digestion is best understood for the Venus flytrap, the most scrutinized of carnivorous plants. If an unwary insect settles on one of its traps and touches one sensory hair, it triggers an electrical signal. If it touches a second hair — proof that it’s prey and not a speck of dirt or dead leaf — then the trap snaps shut.
As the insect struggles and sets off more electrical signals, the trap also begins producing chemicals called jasmonates, which provide the signal to seal the edges of the trap and start filling it with enzymes. As the insect corpse breaks down, the trap ratchets up its output of enzymes and starts production of nutrient transporters, again under the control of jasmonates. It’s a direct steal from the plant defense system, which responds to an insect attack by sending electrical signals to raise the alarm in neighboring cells — which then synthesize jasmonates, which then activate production of defensive proteins.
As it’s ubiquitous to all flowering plants, the jasmonate defense response is a prime candidate for recruitment to the cause of carnivory. In fact, says Renner, “our initial expectation was that the control process might be the same in all carnivorous plants.” And it does turn out to be the same for Nepenthes pitcher plants and sundews as well as flytraps, but those three belong to the same order of plants, so that’s not entirely surprising. Look beyond this trio, however, to the somewhat neglected butterworts, and there’s a tantalizing glimpse of otherness.

Butterworts (Pinguicula) are unshowy plants, with small rosettes of leaves covered in tiny glands that ooze sticky mucilage and digestive enzymes. Most butterworts are entirely passive, although a few can curl the edges of their leaves inward, covering more of the insect in lethal goo. In 2020 the butterwort began to attract a lot more attention following a report from Andrej Pavlovič’s biophysics lab at Palacký University in the Czech Republic.
Pavlovič and his colleagues found that when they fed butterworts a generous helping of fruit flies, the plants responded by churning out enzymes, many of them the same as those identified in other carnivorous plants. So far, so similar. But when it came to the role of jasmonates in switching on production of enzymes, the story was very different.
As in other flowering plants, jasmonates orchestrate the butterwort’s defense against its enemies. Jabbing leaves 10 or 15 times with a needle to mimic attack by an insect prompted a big buildup of jasmonates in the leaves. Prey, on the other hand, triggered almost no response. The team tried another tactic, spraying jasmonate directly onto leaves: In Venus flytraps and sundews that produces a surge of digestive enzymes. In butterworts — zilch.
So butterworts do things differently, although exactly what they do is not yet known. “Butterworts have left us scratching our heads,” says Renner. “The question is, how many other carnivorous plants have figured out their own way?”
If Darwin were here today, he’d pile right in to solve the remaining mysteries of his “most wonderful plants.” He wouldn’t recognize the techniques modern investigators have at their disposal and would be amazed at the quantities of data that can be processed in seconds. But when it comes to designing elegant ways to test theories, he’d be on familiar ground. “Sequencing genomes, counting and analyzing genes are not enough,” says Renner. “You still have to do experiments to find out what genes do, and how they work.”
And that means feeding hungry plants. Darwin fed his on roast meat and hard-boiled egg, cheese, peas and other protein-packed morsels. Today the menu more often consists of less appetizing-sounding “substrate” dosed with precisely measured amounts of nitrogen — but there’s little doubt that Darwin would feel right at home.
This article originally appeared in Knowable Magazine, a nonprofit publication dedicated to making scientific knowledge accessible to all. Sign up for Knowable Magazine’s newsletter.