“Virivores” discovered: Microbes that survive on a virus-only diet
- Viruses are protein-wrapped genetic material that can only replicate within hosts.
- In the first study of its kind, researchers report that certain microbes can eat viruses and grow their populations on a virus-only diet.
- Termed “virivory,” this newly discovered feeding strategy adds a new layer of complexity to food webs.
Viruses are misunderstood. In the shadow of the COVID pandemic, few look kindly on these protein-wrapped jumbles of genetic material, which straddle the murky nexus between the living and non-living.
Though viruses share some common features with living organisms — like possessing a genome and having an ability to replicate — they are not self-sustaining. In other words, to reproduce, viruses depend on infecting host cells. Viruses don’t feed on these cells — indeed, viruses have no metabolism — they simply hijack and reprogram host cells to become miniature factories that produce more virus particles. In the process, they often cause damage or death to the host.
But what if a virus could sustain, rather than decimate, an entire population?
In a new paper published in the Proceedings of the National Academy of Sciences (PNAS), researchers report evidence that microbes can sustain themselves and grow their populations by eating viruses. The breakthrough finding is the first to demonstrate “virivory” — a virus-only diet.
Viruses in the ecosystem
Despite their small size, viruses can have profound impacts on ecosystems. By causing host death, often at a large scale, viruses can affect which organisms survive and which perish. Many ecologists even consider viruses to be a type of predator, perched high atop the food chain (though, as mentioned previously, viruses don’t treat their hosts like “food”).
John DeLong of the University of Nebraska, and lead author of the study, wondered whether viruses could, like other predators, be something else’s prey. DeLong had a specific group of viruses in mind. In 2016, he was part of groundbreaking research investigating chloroviruses (viruses that infect algae in freshwater systems). DeLong figured that, given the abundance of chloroviruses in freshwater, something must be consuming them.
“Everything should want to eat them… Surely something would have learned to eat these really good raw materials,” DeLong said in a statement. Indeed, viruses are a healthy snack. They have plenty of amino acids, as well as nitrogen and phosphorus — the building blocks of a hearty diet.
Finding the virivores
To investigate, DeLong and his team crafted a simple research design. They collected samples of pond water near the University of Nebraska. They isolated different microbes that they thought might consume viruses and added only chloroviruses to the mix, so the microbes would only have virus as a potential food source. Then, they waited to see whose population numbers went up.
Eventually, the researchers narrowed their focus to two genera of protists common in freshwater ecosystems, Halteria and Paramecium. Because these microorganisms inhabit the same habitat as the chloroviruses, it seemed feasible that they had evolved a way to consume the viruses as food. If the researchers could prove that the microbes were growing by eating chlorovirus, they would have compelling evidence that these protists can sustain themselves with a virivorous lifestyle.
Within two days, both Halteria and Paramecium reduced chlorovirus abundance 100-fold, but only Halteria grew its numbers, increasing its population by 15-fold. Halteria converted around 17% of the consumed cholorvirus mass into new mass of its own, a value that is similar to that reported when protists consume bacteria as food. Furthermore, the researchers estimated that each Halteria cell ate about 10,000 to 1,000,000 viruses per day. Scaled up, this means that ciliates in a single pond feasibly could consume ten quadrillion viruses every day in a small pond.
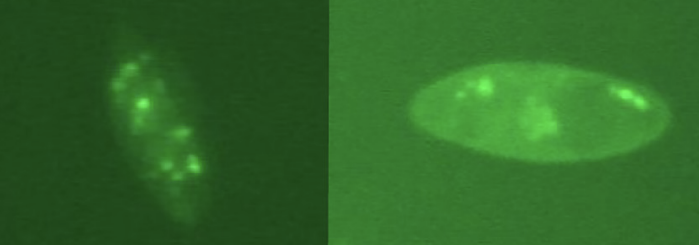
The team also tagged the virus DNA with green fluorescent dye. Under the proper lighting, it could be seen that the vacuoles (sort of like miniature “stomachs” inside the protists) contained chlorovirus.
A new link in the food chain
Food web analysis seeks to understand how energy flows from one organism to another within an ecosystem. Each food chain represents one path nutrients and energy can take as they move through an ecosystem or a more extensive food web. Previously, food web analyses assumed that the resources contained within viruses — carbon, nitrogen, and phosphorous — would remain sequestered and not move up within the food web. In other words, we assumed that viruses “stashed away” the nutrients in particles that nothing else could eat. But this experiment shows that assumption is probably incorrect. This “viral-derived energy,” as the authors write, likely moves up through the aquatic food web and impacts its structure and dynamics.
Protists like Halteria exist toward the bottom of the food chain and serve as important prey for zooplankton. Together, protists and zooplankton represent a considerable fraction of living biomass and contribute vast amounts of energy to the food web. Current models do not include the trophic link between viruses and their consumers, thus ignoring a critical interaction and miscalculating the trophic transfer of energy throughout a given ecosystem.
Since the study was completed, DeLong and his team have found other ciliates that can thrive on a virus-only diet. Yet, the researchers still need to prove that virivory exists outside the lab in the wild. If it does, which seems likely, the discovery could revolutionize our understanding of microbial ecosystems.