Unexpected protein found in diseased brains

- Protein fibrils accumulate in the brain during neurodegeneration.
- Research has suggested the protein TDP-43 forms fibril aggregates, possibly leading to frontotemporal lobar degeneration.
- Now, cryo-electron microscopy has revealed the presence of a previously unknown protein fibril in diseased brains.
The brain is, perhaps, the worst place to find an unexpected clump of proteins, as they are the telltale sign of neurodegenerative disease. As insoluble proteins aggregate together in the brain, forming long, hair-like fibrils, neurological function declines.
Although the role of these fibrils is a mystery, researchers and physicians use them to characterize diseases. Furthermore, knowing the precise structure of fibril-forming proteins is critical for developing effective therapies — including vaccines — for neurodegenerative diseases. For example, amyloid-β fibrils form in Alzheimer’s disease. In 2021, a group of researchers discovered a special pattern on the proteins that allowed them to develop a vaccine against an Alzheimer’s-like disease in mice.
Hoping to find new ways of treating neurodegenerative disorders, two independent groups of scientists — one led by David Eisenberg and the other by Sjor Scheres and Michel Goedert — investigated the structure of the proteins involved in a type of dementia called frontotemporal lobar degeneration (FTLD). They discovered the presence of a previously unknown protein fibril in the brain. While both groups identified the same protein through similar techniques, they came to markedly different conclusions about its relevance.
TDP-43: An expected protein in diseased brains
Half of all FTLD cases are characterized by insoluble deposits of a DNA binding protein called TDP-43. As Derek Lowe, writer for Science, puts it, “TDP43 itself is an interesting beast.” Despite its name, TDP-43’s RNA-binding profile is far more impressive than its DNA-binding. According to previous studies, the protein can associate with more than 6,000 different RNA targets, which is nearly 30% of all human RNA. Additionally, the protein interacts with a few heavy hitter proteins, including nuclear factor kappa B (an ancient regulator of the innate immune system) and a couple of heat shock proteins (which prevent other proteins from falling apart under stressful conditions).
Consequently, it is little suprise that this protein is found in diseased brains. Scientists have found TDP-43 aggregates in four neurodegenerative diseases: FTLD, amyotrophic lateral sclerosis (ALS), primary lateral sclerosis, and progressive muscular atrophy. The structure of the TDP-43 fibrils, however, has never been determined.
Trying to resolve TDP-43 structures in the brains of FTLD patients (and discover avenues of treatment), Eisenberg’s team used a method called cryo-electron microscopy (cryo-EM). Although cryo-EM is a decades-old technique, it has gained increasing interest among molecular biologists and biochemists. In 2013, a series of technological and algorithmic breakthroughs — described as the “resolution revolution” — significantly improved the resolution obtainable by this technique. In 2020, researchers used this technology to locate individual atoms within a protein for the first time.
Scheres’s team also used cryo-EM in their study, but they weren’t interested in TDP-43. Instead, they were studying a protein called TMEM106B. As it turned out, Eisenberg’s team was also studying TMEM106B; they just didn’t know it yet.
TMEM106B: An unexpected protein in diseased brains
TMEM106B is a protein involved in the cells’ waste management system. About a decade ago, scientists found that certain genetic variations of TMEM106B increased the risk of developing FTLD. However, experiments have failed to detect TMEM106B fibrils in diseased brains. Scheres and Eisenberg’s teams, however, did find the fibrils, even though they weren’t looking for them.
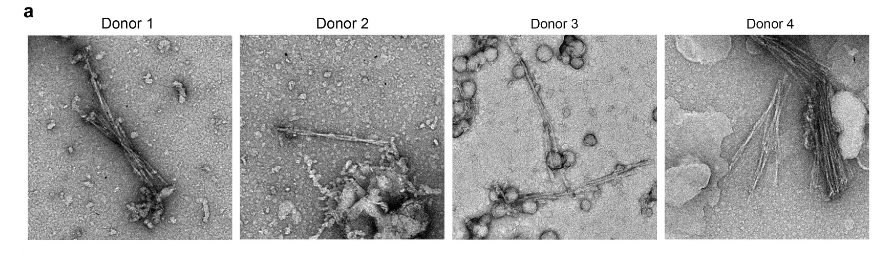
Eisenberg’s team isolated the fibrils found in the brains of four patients with FTLD, believing the fibrils were primarily composed of TDP-43. As they analyzed the proteins’ atomic structure, however, it became clear that they had identified something unknown. The researchers compared their unknown structure to other human proteins and found only one match: TMEM106B, which had never been identified in fibrils before. They also found abundant TDP-43, but it didn’t form the fibril-like clumps associated with neurodegeneration. Thus, the team concluded TMEM106B fibrils are a characteristic FTLD.
Scheres’s team also found TMEM106B fibrils in the brains of people who had died of FTLD, as well as a range of other neurodegenerative diseases, including ALS. However, they also found the fibrils in the brains of healthy older individuals. Ergo, they concluded that TMEM106B fibrils build-up during aging and may not be relevant to disease.
Opening new avenues for investigation
Even if TMEM106B fibrils do not cause disease, their discovery certainly is not meaningless. The risk of developing a neurodegenerative disease increases with age. If TMEM106B fibrils accumulate with age, then it is possible that the build-up of TMEM106B fibrils drives neurodegeneration via other factors. Regardless of TMEM106B’s role in causing disease, this structure opens new avenues for investigation.