How horseshoe crab blood became one of the most valuable liquids in medicine

- Horseshoe crabs are not only disease-resistant but also have an impressive ability to survive extreme physical damage.
- The main reason centers on a unique and ancient immune defense mechanism: a special type of blood cell called an amoebocyte, which causes the crabs’ blood to clot into stringy masses when it encounters endotoxins.
- In the 1970s, the medical industry began using this special clotting component to test for the presence of bacteria on medical devices and within vaccines.
Excerpted from Pump: A Natural History of the Heart © 2021 by Bill Schutt. Reprinted by permission of Algonquin Books of Chapel Hill.
The story of the Atlantic horseshoe crab’s first turn toward medical relevance occurred in 1956. That’s when Woods Hole pathobiologist Fred Bang determined that certain types of bacteria caused horseshoe crab blood to clot into stringy masses. He and his colleagues hypothesized that this was an ancient form of immune defense. Eventually, they determined that a type of blood cell called an amoebocyte was responsible for the clot formation. As their name implies, amoebocytes resemble amoebas, the blobby single-celled protists that make pseudopods so popular and dysentery so unpopular.
Bang, and those who followed up his research, hypothesized that the clotting ability of the amoebocyte evolved in response to the bacteria-and-pathogen-rich muck that horseshoe crabs plow through for pretty much their entire lives. Their army of blood-borne amoebocytes can wall off foreign invaders, isolating them in prisons of gelatinous goo before they can spread their infections.
As a result, horseshoe crabs are not only disease-resistant but have an impressive ability to survive extreme physical damage. The most lethal-looking wounds are quickly plugged with amoebocyte-generated clots, allowing banged-up individuals to carry on as if they hadn’t just lost a fist-sized section of shell to an outboard motor propeller. This unique defense-and-repair system may be at least partially responsible for the horseshoe crabs’ record of having been around for nearly half a billion years, a period during which they’ve survived a total of five planetwide extinction events.
We now know that the amoebocytes do their thing by detecting potentially lethal chemicals called endotoxins. These are associated with gram-negative bacteria, a class of microbes that includes pathogens like Escherichia coli (food poisoning), Salmonella (typhoid fever and food poisoning), Neisseria (meningitis and gonorrhea), Haemophilus influenzae (sepsis and meningitis), Bordetella pertussis (whooping cough), and Vibrio cholerae (cholera).
Oddly, the endotoxins are not themselves responsible for the myriad diseases associated with these bacteria. Nor are they protective products—released, for example, to combat the bacteria’s own enemies. Instead, these large molecules form much of the bacterial cell membrane, helping to create a structural boundary between the cell and its external environment. Endotoxins are also known as lipopolysaccharides, since they consist of a fat attached to a carbohydrate. These molecules become problematic for other organisms only after the bacteria have been killed and sliced open, or lysed—something that can happen when the immune system (or an antibiotic) is engaged to fight off a gram-negative bacterial infection. At this point, the bacterial cell contents spill out and the lipopolysaccharide components of the membrane are released into the environment.
Unfortunately, although the disease-causing bacteria may have been conquered, the sick host’s problems are not over. The presence of endotoxins in the blood can cause the rapid onset of fever, one of the body’s protective responses to a foreign invader. Such fever-inducing substances are called pyrogens, and they can lead to serious problems (like brain damage) if they drive body temperatures too high for too long. Further complications can also arise from the body’s dangerously overblown immune response—a condition healthcare professionals have been forced to deal with during the coronavirus pandemic. In the worst cases, exposure to endotoxins can lead to a condition known as endotoxic shock, a cascade of life-threatening symptoms that range from damage to the lining of the heart and the blood vessels, to dangerously low blood pressure.
After our trip to find horseshoe crab eggs at the beach, Leslie and I accompanied Dan Gibson to the Woods Hole lab, where he prepared a microscope slide of fresh horseshoe crab blood. We were soon examining live horseshoe crab amoebocytes.
“They’re all full of granules,” I said, noting the sand-like particles that packed the cell interiors.
“Those are tiny packets of a protein called coagulogen,” Gibson said. As their name may suggest, coagulogens cause coagulation, or clotting. “When the amoebocytes encounter even the slightest amount of endotoxin, they release their packets of coagulogen, which quickly transforms into a gel-like clot.”
Because endotoxins can cause such a dangerous response in humans, in the 1940s the pharmaceutical industry began to test its products for the presence of these substances, which can also be released by accident during the drug manufacturing process. One of the first methods developed was the rabbit pyrogen test, which became an industry standard. Here’s how it worked: In what definitely sounds like a job for “the new guy,” baseline rectal temperatures were taken for lab rabbits involved in the test. Next, the lab technicians injected rabbits with the batch of whatever drug was being tested, often doing so via an easily accessible ear vein. They then recorded rectal temperatures every thirty minutes for the next three hours. If a fever developed, it would signal the potential presence of an endotoxin in that particular batch.
Having discovered that horseshoe crab blood would clot in the presence of endotoxins, in the late 1960s, a colleague of Fred Bang’s, hematologist Jack Levin, developed a chemical test, known as an assay, that would come to replace the laborious and controversial rabbit pyrogen test. Essentially, Levin and his colleagues sliced open horseshoe crab amoebocytes to collect the clot-forming component, a substance they named Limulus amoebocyte lysate (LAL). Not only could LAL be used to test for the presence of endotoxins in batches of pharmaceuticals and vaccines, researchers eventually discovered that it also worked on instruments like catheters and syringes, medical devices for which sterilization might kill bacteria but also could accidentally introduce endotoxins into patients receiving medical care.
While this discovery was presumably greeted by relief within the rabbit community, horseshoe crabs and their fans were somewhat less than thrilled, especially when another Woods Hole researcher quickly established a biomedical company that began extracting horseshoe crab blood on an industrial scale. Three more such companies soon sprang up along the Atlantic coast, turning the production of LAL into a multimillion-dollar industry. As a result, today nearly half a million horseshoe crabs are hauled out of the water each year, many during spawning season. Most are transported to industrial-sized lab facilities, not in tanks of cold salt water, but in the back of open pickup trucks. Upon arrival, the crabs encounter teams of mask- and gown-clad workers, who scrub them with disinfectant, bend their hinged shells in half (“the abdominal flexure position”), and strap them to long metal tables, assembly line–style. Large-gauge syringes are then inserted directly into the horseshoe crabs’ hearts. The blood, blue-tinted and with the consistency of milk, drips down into glass collecting bottles. And in a move that that would make Count Dracula envious, the collection continues until the blood stops flowing, usually when around 30 percent of it has been drained.
In theory at least, the horseshoe crabs are supposed to survive their ordeal, and once bled, by law they must be returned to the approximate area where they were collected. But according to Plymouth State University neurobiologist, Chris Chabot, an estimated 20 to 30 percent of the crabs die during the roughly seventy-two hours from collection to bleeding to return.
“It’s significant that the gill-breathing crabs are held out of the water for the entire time,” Chabot told Leslie and me. We were visiting the scientist and his colleague, zoologist Win Watson, at the University of New Hampshire’s Jackson Estuarine Laboratory.
Also of potential significance, Chabot explained, is the fact that no one knows whether previously bled specimens suffer any short- or long-term effects after being returned to the water—or even whether they survive. (The Atlantic States Marine Fisheries Commission [ASMFC] has been formally managing horseshoe crab populations since 1998, but various policies have hampered its ability to access mortality rate numbers in horseshoe crabs harvested for biomedical companies.) With this in mind, Chabot and his research team have been trying to determine the effect that the harvesting process has on horseshoe crabs once they are returned to the water. To do this, he and his students collected a small number of specimens and subjected them to conditions mimicking those the crabs face during encounters with the biomedical industry.
Chabot and his students observed listlessness and disorientation in their subjects, which they hypothesized was due in part to the fact that after bleeding, the crab’s body can’t deliver as much oxygen as it requires. “It takes weeks to replenish the amoebocytes and the hemocyanin they’ve lost,” he told us.
Chabot also explained that with many of their protective amoebocytes being lysed in a test tube somewhere, things like wound repair and a return to environments infested with gram-negative bacteria made for a pretty grim outlook for those horseshoe crabs headed home after a long day on the assembly line.
Watson confirmed that the combination of three days spent out of the water, at high temperatures, coupled with significant blood loss, can make for a lethal combination for horseshoe crabs. What’s more, he added, since crabs are usually collected during the mating season, and often before mating occurs, any death rate would have the potential to affect the size of future generations—especially since the larger female crabs are preferentially selected during collection. And given that the crabs have slow maturation times, the extent of the problems that are brewing may not become apparent to researchers, or anyone else, for a decade. According to the ASMFC, the New York and New England regions are already starting to see a decrease in the abundance of horseshoe crabs.
Watson and Chabot both suggested that some fairly simple steps could be undertaken to improve mortality numbers, thus helping sustain horseshoe crab populations without hurting the LAL industry. The first step would be to delay the harvesting of horseshoe crabs until after the mating season. Their second suggestion was to transport specimens to and from biotech labs in cool water tanks rather than stacking them up, dry and hot, on boat decks and in the backs of trucks. This, the horseshoe crab mavens explained, would not only prevent heat stress but also keep the thin, membranous “pages” of their book gills from drying out.
From talking to Watson and Chabot, it is clear to me that they fully appreciate the importance of LAL to the medical community and to the patients whose lives it saves. These researchers are simply trying to improve the odds for a species that has been coping with threats to its existence long before humans showed up and added pollution, habitat destruction, and overharvesting to the horseshoe crab shit list.
Although the steps Watson and Chabot suggested would go a long way toward improving horseshoe crab mortality, there is another harvesting-related risk. This one stems from the fact that each horseshoe crab heartbeat is initiated and controlled by a small mass of neurons called a ganglion, located just above the heart. Its job is to stimulate each section of the heart to contract in the right order in response to minute electrical pulses.
These neurogenic hearts are found in crustaceans like shrimp as well as segmented worms like earthworms and leeches. They differ significantly from the myogenic hearts seen in humans and other vertebrates, which beat without being stimulated by external structures like ganglia or nerves. Instead, the stimulus for myogenic contraction originates in small regions of specialized muscle tissue called cardiac pacemakers, located within the heart itself.
The absence of these pacemakers in neurogenic hearts may at least partially explain why Aztec art never depicts priests as holding the still-beating hearts of newly sacrificed lobsters or horseshoe crabs. That’s because their neurogenic hearts would have stopped beating the moment they were severed from the ganglia controlling them.
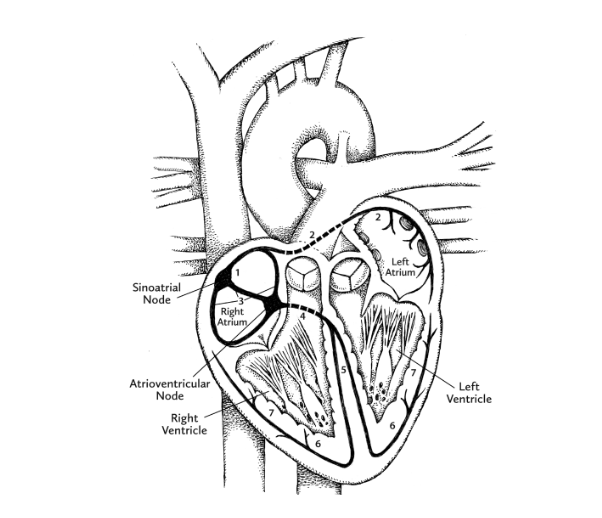
Meanwhile, thanks to pacemaker cells, human hearts have the ability to generate a continuous sequence of electrical signals. These begin at a location in the right atrium called the sinoatrial (SA) node, and speed through the heart along highly specific routes called conduction pathways. Moving like ripples of water after the splash of a pebble, the signals travel from the right atrium to the left atrium, both situated within the uppermost “base” of the heart. As the ripple begins to move downward toward the ventricles, another patch of pacemaker cells, called the atrioventricular (AV) node, slows down the signal, the slight lag time allowing the ventricles to fill with blood. The electrical signal from the AV node continues down toward the pointy apex of the heart. As it does, the muscles making up each ventricle are stimulated to contract in turn.
But while our myogenic heart initiates its own beat, a pair of nerves control the rate and the strength of contraction. These are the vagus nerve, which slows down the heartbeat, and the cardiac accelerator nerve, which . . . well, you know. They work as part of the autonomic nervous system (ANS), which goes about its considerable duties without your consent or voluntary input.
There are two divisions of the ANS. One, the sympathetic division, prepares you to deal with real or imagined threats with a host of responses, including increased heart rate and blood pressure. This is often referred to as the “fight-or-flight response.” As your heart rate speeds up, your ANS also causes an increase in blood flow to your brain and leg muscles. This occurs as blood vessels supplying those areas receive a signal to start vasodilation (i.e., widening of their inner diameters). Simultaneously, blood is diverted away from the digestive tract and kidneys through vasoconstriction of the tiny blood vessels that normally supply them. The reasoning here is that digesting Cheerios and producing urine becomes somewhat less important when you are suddenly confronted by a grizzly bear or the prospect of speaking in front of an audience. Instead, the extra blood heads to the leg muscles through their wide-open capillaries—preparing you for a sprint. The blood flow is also increased to the brain, presumably enabling you to figure out what to do if running away doesn’t work.
The second division of the autonomic nervous system is the parasympathetic division, which takes over during normal (a.k.a. grizzly bear- and public-speaking-free) conditions. This is the “rest-and-repose” alternative of the ANS. It slows down the heart rate, directing blood flow to the organs slighted by the fight-or-flight response, like those that handle digestion and urine production.
Interestingly, if the nerves that control the ANS are damaged, or if their impulses are blocked (attention fugu fans), the heart does not stop beating—which would be quickly fatal. Instead the SA node takes over regulation of the heart rate, setting the pace internally at around 104 beats per minute.
The problem for a horseshoe crab getting the hypodermic Dracula treatment is that its heart has no such ability to pace itself. Its heartbeat is solely governed by the ganglion situated above it.
Watson explained that the ganglion activates motor neurons, which communicate with the heart muscle by releasing a neurotransmitter called glutamate. This chemical messenger fits like a key into neurotransmitter-specific locks found on the surface of the heart. These locks are known as receptors, and the resulting lock-and-key arrangement directs the cells making up that muscle to contract.*
“The problem is,” Watson said, “that if you stick a needle into a horseshoe crab to drain its blood and you hit the cardiac ganglion by mistake, you’ll likely kill the animal.”
“So, workers bleeding specimens in these biomedical facilities have to take the location of the cardiac ganglion into consideration when they insert their needles, right?”
Watson shook his head. “Bill, I doubt any of them even know about it.”