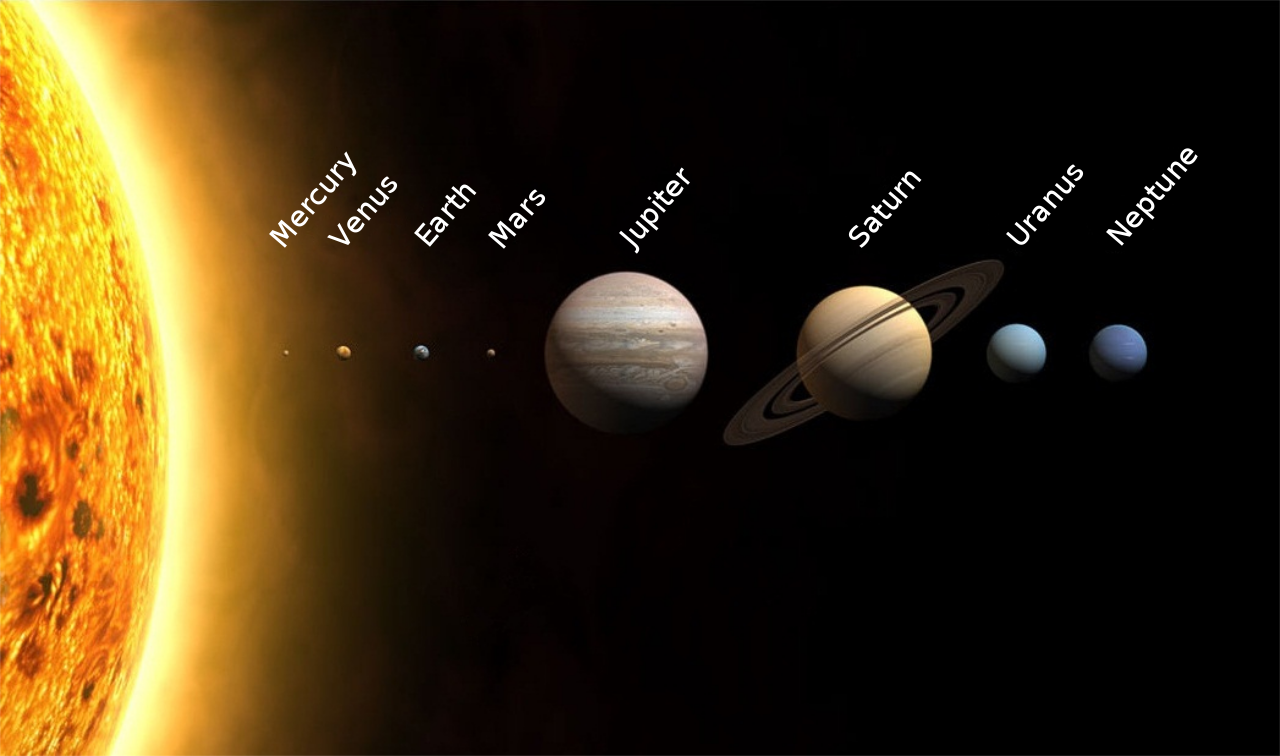
Why Mercury could be encrusted with diamonds
- Mercury is unique among the rocky planets, having formed a graphite shell early in its history.
- This high concentration of carbon may have been converted to diamonds via meteor impacts over the course of billions of years.
- Confirmation of diamonds on the surface of Mercury may come in a few years, with the BepiColombo mission.
Last month at the Lunar and Planetary Science Conference in the Woodlands, Texas, Dr. Kevin Cannon of Colorado School of Mines presented surprising details on the composition of the planet Mercury. Cannon showed that Mercury’s history of being pummeled with meteors, combined with some of its unique properties, may potentially cause the planet to be “encrusted in diamonds.”
Planet Bling
The story of Mercury’s unique composition began when Mercury first formed. As with many rocky planets, the crust of Mercury formed by crystallizing out of a magma ocean. On Mercury, however, this shell was different than other rocky planets in our solar system: It was composed of carbon, forming a graphite “shell.” We don’t see a carbon shell elsewhere in our solar system because of some unique properties of Mercury, namely, the fact that it lacks an atmosphere.
Mercury is small in comparison to Earth, being just over a third of its radius. In addition, its proximity to the Sun blasted the tiny planet with radiation. These two effects caused most of Mercury’s atmosphere to be lost to space.
This, in turn, went on to influence the composition of the crust. Because Mercury was not able to hold onto its atmosphere, it lost the key element oxygen. On Earth, oxygen combined with other elements, like hydrogen, nitrogen, and carbon. On Mercury, however, there was likely very little oxygen, and this changed everything.
“Carbon could crystallize as pure graphite and float to the surface instead of combining with oxygen to form molecules like carbon dioxide or carbon monoxide,” Cannon told Big Think. In Mercury’s early history, this shell of carbon could potentially be quite thick. Models predict it could reach a depth of anywhere from one meter to 21 kilometers.
Over billions of years, its lack of atmosphere could not protect the planet from being pummeled by meteors. On Earth, our atmosphere slows down and burns up many incoming space rocks. But Mercury, much like our Moon, is marked by countless craters. These meteors hit the surface with tremendous force — enough to generate diamonds.
Forming diamonds on Mercury
Cannon wanted to see what happened over the course of 4.5 billion years as Mercury was bombarded by meteors. To do this, he assumed several depths of the initial graphite shell, ranging from 100 to 500 meters deep. He then ran simulations of meteorites impacting Mercury. It didn’t take long for about one-third of the graphite to be converted into diamonds, independent of how deep the graphite shell was.
Indeed, certain kinds of stony meteorites, called ureilites, have been known to contain diamonds. Some studies have suggested that these diamonds formed during impacts. “We can be almost certain the diamonds formed by asteroids colliding in space,” says Cannon. But they would not have formed on Earth. “The small meteorites we find have been slowed down by the atmosphere to terminal velocity, so they don’t hit with enough speed to generate the pressures needed to form diamond,” he said.
Instead, on Earth diamonds are formed deep underground. Within the mantle, at a depth of about 150 kilometers, pressures and temperatures are high enough to forge diamonds. These diamonds are later brought to the surface during volcanic eruptions.
As time goes on, the surface of Mercury would be churned up. Impacts would mix upper layers of carbon with lower layers of silicon in minerals such as feldspar or pyroxene. Lava flows would also cover the graphite. But the diamonds are likely to persist, even if they are buried. Diamonds melt at 4,027 C° (7,281° F), so it takes a lot to destroy them once they form.
Diamond hunters
Why haven’t we seen diamonds on Mercury’s surface before? MESSENGER, which orbited Mercury for four years, did not detect these diamonds. However, this could be because MESSENGER imaged the surface in the near-infrared, where diamonds have no spectral signature. In 2025, when the BepiColombo mission reaches Mercury, it may be able to detect them.