Asymmetric organocatalysis: The simple chemistry discovery that won the 2021 Nobel Prize
- The chemists Benjamin List and David MacMillan were awarded the 2021 Nobel Prize in chemistry.
- List and MacMillan, working independently of each other, discovered an environmentally friendly way of accelerating and controlling chemical reactions.
- Called asymmetric organocatalysis, the discovery has led to a growing field of research on how simple organic molecules can do things within chemical manufacturing that other catalysts cannot.
The 2021 Nobel Prize in Chemistry was awarded to chemists Benjamin List and David MacMillan for their work on asymmetric organocatalysis. The term describes a method for accelerating chemical reactions and creating specific types of molecules. List and MacMillan’s work, which they conducted independently of each other and published in 2000, has helped to advance pharmaceutical research and make the production of chemicals more efficient and less harmful on the environment.
Understanding asymmetric organocatalysis
While asymmetric organocatalysis may sound complicated, we can break it down in simple English. First, the “catalysis” of organocatalysis. To catalyze something is to cause something to occur or to increase the rate at which it occurs. Eating donuts catalyzes weight gain. Drinking coffee catalyzes alertness (and also anxiety and jitters). Catalysis is the process of creating and increasing something; it is the action of catalyzing.
In chemistry, catalysis refers to catalyzing the rate of a chemical reaction by adding another substance: the catalyst. Donuts and coffee were the catalysts above. A relatively small amount of a catalyst can increase the rate and efficiency of most chemical reactions. As such, catalysts are extremely important in the multibillion-dollar chemical manufacturing industry; almost all chemical products undergo a catalytic process.
A new type of catalyst
Until the discovery of asymmetric organocatalysis, chemists had believed there were only two categories of catalysts: metals and enzymes.
The vast majority of chemical catalytic processes require metals, often transition metals. For example, electrolysis of water to produce hydrogen often uses nickel or platinum metal as a catalyst. The catalytic convertor on your car uses platinum, palladium, or rhodium to catalyze the reaction of carbon monoxide and hydrocarbons in combustion exhaust gas with oxygen to form carbon dioxide and water. Nickel is used to produce vegetable oil. Although metals are effective catalysts, they’re also often toxic for people and the environment.
Nature also uses catalysts. Nearly all metabolic processes in living cells require enzymes to keep reaction rates high enough to remain alive. Digestion, muscle contraction, DNA replication, and fermentation all rely upon enzymes. These processes can be summed up in chemistry jargon as examples of “biocatalysis.” Enzymes make good catalysts in the body, but they can difficult to work with in the lab.
List and MacMillan, among others, established an entirely new type of catalysis, adding organocatalysis to the two existing categories: metal catalysis and natural biocatalysis. A 2014 review described how organocatalysis has helped make medicinal chemistry safer and more efficient:
“In general, organocatalysts are air- and moisture-stable and, thus, inert-equipments such as vacuum lines or glove boxes are not necessary. They are easy to handle even on large scale and relatively less toxic compared to transition metals. Moreover, frequently the reactions are conducted under mild conditions and high concentrations thus avoiding the use of large amounts of solvents and minimizing waste.”
The final part of understanding this Nobel Prize is to tack on the ‘asymmetric’ to the organocatalysis.
Adding the “asymmetry”
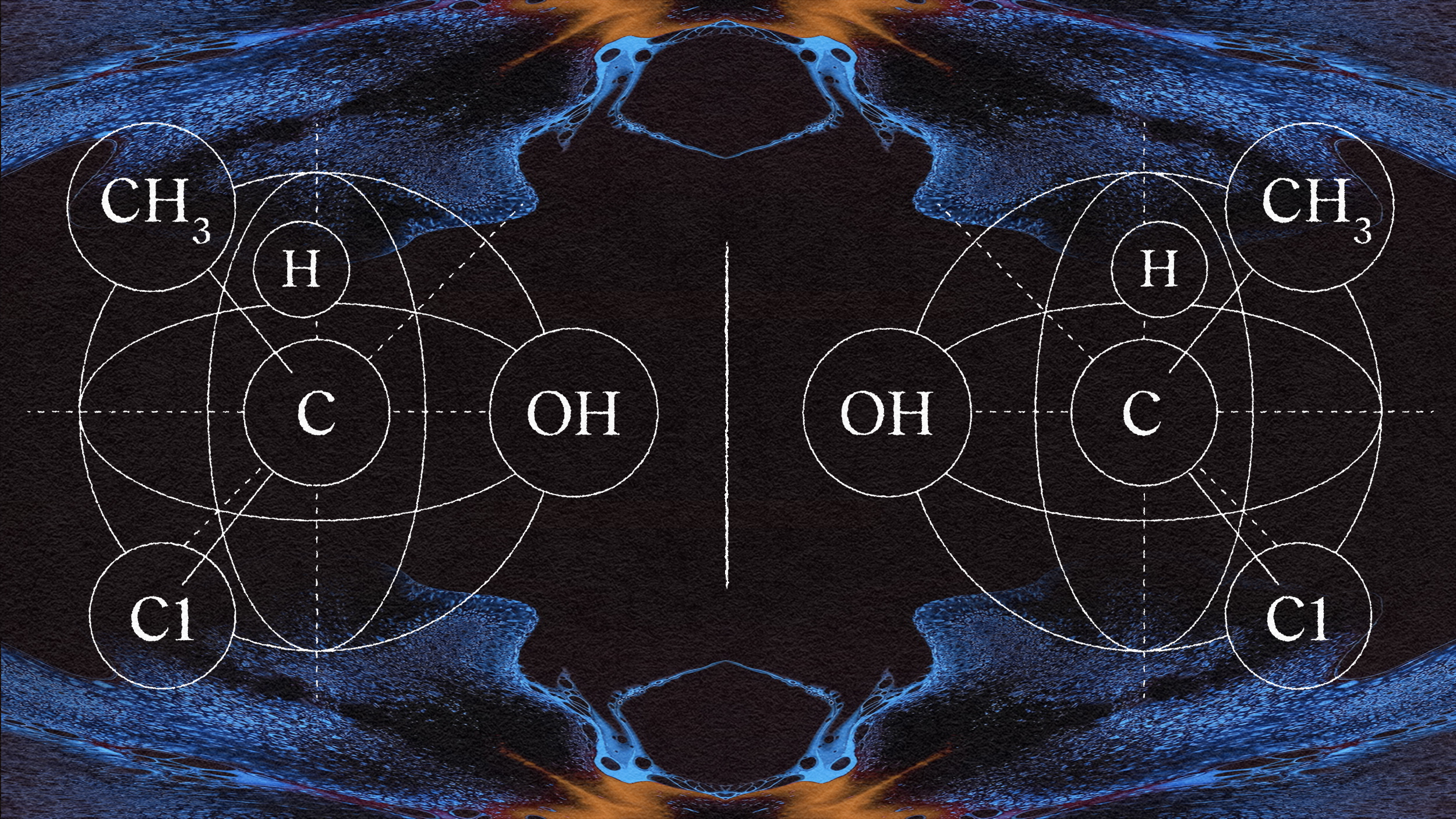
Some organic molecules possess an interesting property: the molecule has a so-called mirror image that can behave differently. To understand this, chemists often use the example of the identical physiology of the left and right hand. All of the fingers are connected in just the same way, and one can be laid on top of the other, but they are not the same. They can’t be interchanged.
This property is called chirality, a term derived from “kheir,” which is Greek for “hand.” The chirality of a molecule can have dramatic consequences for how it reacts to other molecules. For example, one of the two chiralities of thalidomide is an effective commercial sedative and cancer medication. The other causes terrifying birth defects.
Here’s another example of chirality, found in the molecule limonene, whose mirror images produce the scent of either lemon or orange.
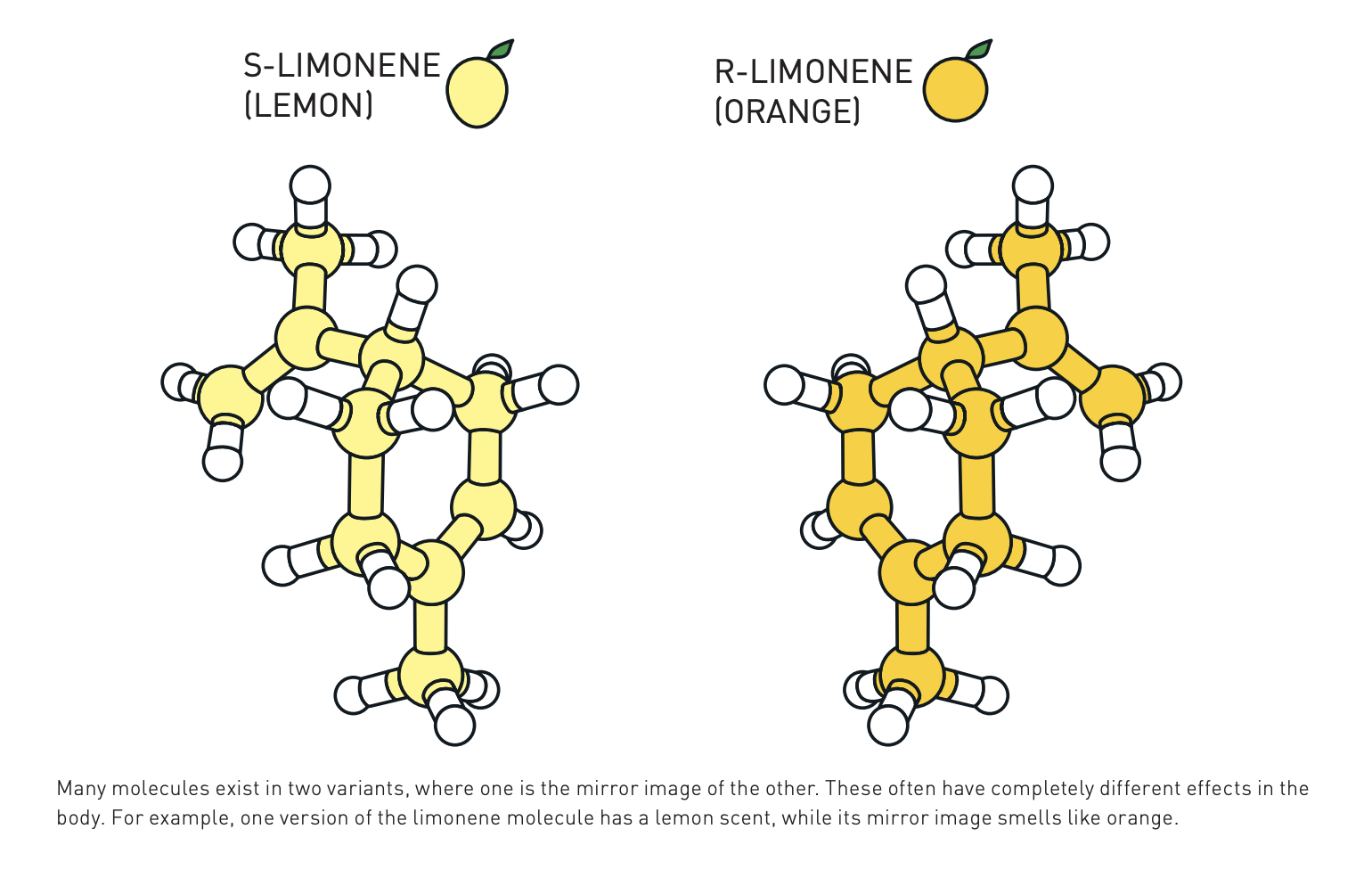
In general, chiral molecules react with other chiral molecules in different ways, depending on whether each molecule is right- or left-handed. In general, a left-handed molecule put to pharmaceutical or other organic uses will be an entirely different drug, with different effects, than its right-handed analog. The organocatalysis that List and MacMillan developed can be used to encourage reactions that specifically produce one of the two mirror images. So, in this application, it is uneven — or asymmetric — organocatalysis.
Since 2000, asymmetric organocatalysis has sparked a growing field of research into how simple organic molecules can do things within chemical manufacturing that traditional catalysts cannot, in
One more thing
This award should hearten anyone who’s working toward uncertain goals. When notified of winning the most prestigious prize in the world, List said: “I literally felt like I was the only one working on this. […] I thought, maybe it’s a stupid idea, or maybe someone has tried it already.” Just like the rest of us, Nobel Prize winners are fumbling in the dark, alone and uncertain, looking for something that they’re not even sure makes sense.