How to make peace with the weirdness of quantum mechanics
- The laws of physics always apply to every object in the Universe at all times, but on quantum scales, the behavior is far from intuitive.
- At a fundamentally quantum level, everything is both wave and particle with an indeterminate state until measured, where measurement outcomes can only be predicted probabilistically.
- Still, it’s the most successful, most powerful framework ever developed to describe reality, and everything in existence, without exception, obeys its rules.
The most remarkable fact about the Universe is simply that it can be understood at all. The universe, for all its complexity, can be reduced to its simplest, most fundamental components. If you can determine the underlying rules, laws, and theories that govern your reality, then as long as you can specify what your system is like at any moment in time, you can use your understanding of those laws to predict what things will be like both in the far future as well as the distant past. The quest to unlock the secrets of the universe is fundamentally about rising to this challenge: figuring out what makes up the universe, determining how those entities interact and evolve, and then writing down and solving the equations that allow you to predict outcomes that you have not yet measured for yourself.
It’s an idea that makes perfect sense, and applies very well to our classical notions of reality. Newton, Maxwell, and even Einstein would all be quite happy with these ideas, but when it comes to our quantum reality — including the questions of what makes up the Universe and how the laws of nature work in practice — the picture of reality that emerges is far less clear. In fact, quantum mechanics is arguably the most counterintuitive picture of reality we’ve ever discovered; we would never have concocted it if experiments and observations didn’t compel us to. Still, with a little change to your way of thinking, you can begin to make sense of, or at least make peace with, the quantum nature of our reality.
Before there was quantum mechanics, we had a series of assumptions about the way the universe worked. We assumed that everything that exists was made out of matter, and that at some point, you’d reach a fundamental building block of matter that could be divided no further. In fact, the very word “atom” comes from the Greek ἄτομος, which literally means “uncuttable,” or as we commonly think about it, indivisible. These uncuttable, fundamental constituents of matter all exerted forces on one another, like the gravitational or electromagnetic force, and the confluence of these indivisible particles pushing and pulling on one another is what was at the core of our physical reality.
The laws of gravitation and electromagnetism, however, are completely deterministic. If you describe a system of masses and/or electric charges, and specify their positions and motions at any moment in time, those laws will allow you to calculate — to arbitrary precision — what the positions, motions, and distributions of each and every particle was and will be at any other moment in time. From planetary motion to bouncing balls to the settling of dust grains, the same rules, laws, and fundamental constituents of the universe accurately described it all.
Until, that is, we discovered that there was more to the universe than these classical laws.
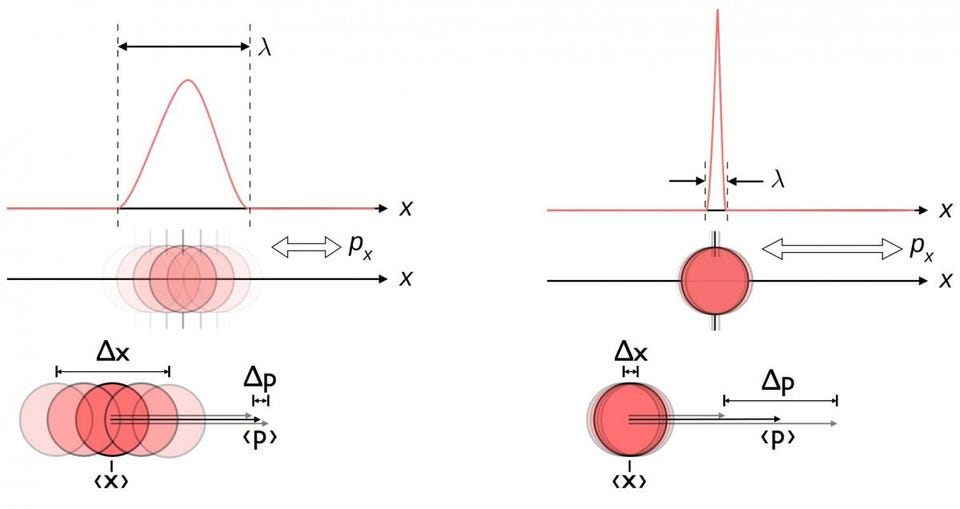
1.) You can’t know everything, exactly, all at once. If there’s one defining characteristic that separates the rules of quantum physics from their classical counterparts, it’s this: you cannot measure certain quantities to arbitrary precisions, and the better you measure them, the more inherently uncertain other, corresponding properties become.
- Measure a particle’s position to a very high precision, and its momentum becomes less well-known.
- Measure the angular momentum (or spin) of a particle in one direction, and you destroy information about its angular momentum (or spin) in the other two directions.
- Measure the lifetime of an unstable particle, and the less time it lives for, the more inherently uncertain the particle’s rest mass will be.
These are just a few examples of the weirdness of quantum physics, but they’re sufficient to illustrate the impossibility of knowing everything you can imagine knowing about a system all at once. Nature fundamentally limits what’s simultaneously knowable about any physical system, and the more precisely you try to pin down any one of a large set of properties, the more inherently uncertain a set of related properties becomes.
2.) Only a probability distribution of outcomes can be calculated: not an explicit, unambiguous, single prediction. Not only is it impossible to know all of the properties, simultaneously, that define a physical system, but the laws of quantum mechanics themselves are fundamentally indeterminate. In the classical universe, if you throw a pebble through a narrow slit in a wall, you can predict where and when it will hit the ground on the other side. But in the quantum universe, if you do the same experiment but use a quantum particle instead — whether a photon, an electron, or something even more complicated — you can only describe the possible set of outcomes that will occur.
Quantum physics allows you to predict what the relative probabilities of each of those outcomes will be, and it allows you to do it for as complicated of a quantum system as your computational power can handle. Still, the notion that you can set up your system at one point in time, know everything that’s possible to know about it, and then predict precisely how that system will have evolved at some arbitrary point in the future is no longer true in quantum mechanics. You can describe what the likelihood of all the possible outcomes will be, but for any single particle in particular, there’s only one way to determine its properties at a specific moment in time: by measuring them.
3.) Many things, in quantum mechanics, will be discrete, rather than continuous. This gets to what many consider the heart of quantum mechanics: the “quantum” part of things. If you ask the question, “How much?” in quantum physics, you’ll find that there are only certain quantities that are allowed.
- Particles can only come in certain electric charges: in increments of one-third the charge of an electron.
- Particles that bind together form bound states — like atoms — and atoms can only have explicit sets of energy levels.
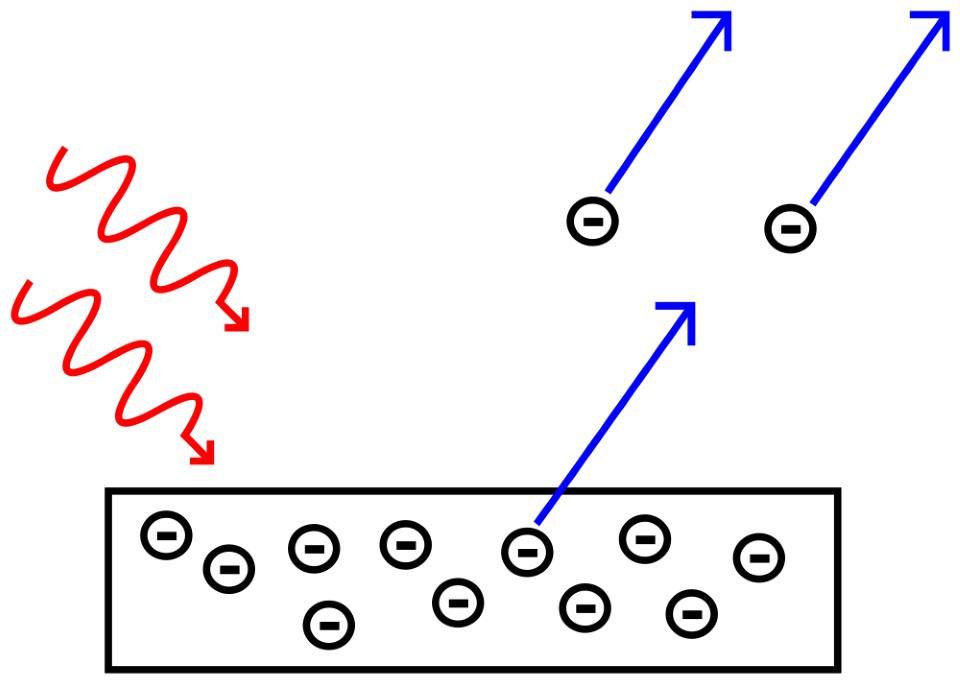
- Light is made up of individual particles, photons, and each photon only has a specific, finite amount of energy inherent to it.
In all of these cases, there’s some fundamental value associated with the lowest (non-zero) state, and then all other states can only exist as some sort of integer (or fractional integer) multiple of that lowest-valued state. From the excited states of atomic nuclei to the energies released when electrons fall into their “hole” in LED devices to the transitions that govern atomic clocks, some aspects of reality are truly granular, and cannot be described by continuous changes from one state to another.
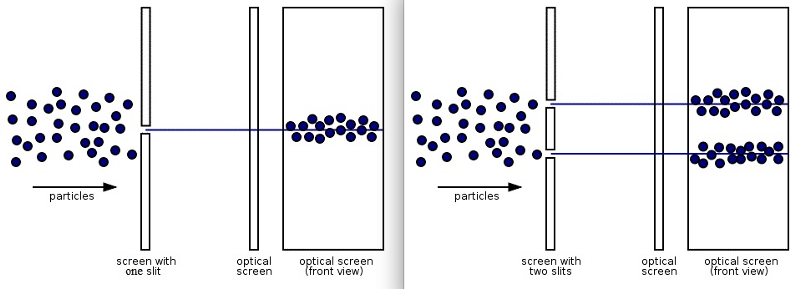
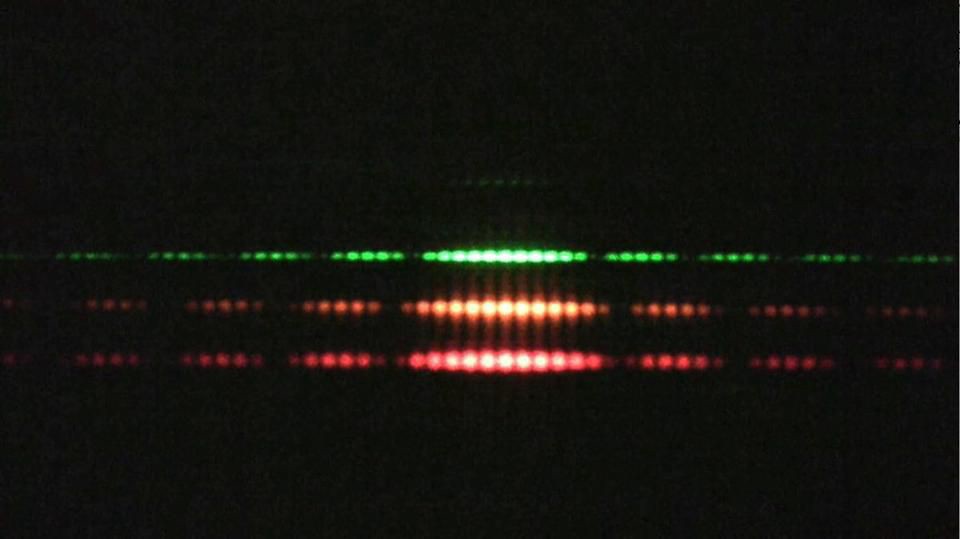
4.) Quantum systems exhibit both wave-like and particle-like behaviors. And which one you get — get this — depends on if or how you measure the system. The most famous example of this is the double slit experiment: passing a single quantum particle, one-at-a-time, through a set of two closely-spaced slits. Now, here’s where things get weird.
- If you don’t measure which particle goes through which slit, the pattern you’ll observe on the screen behind the slit will show interference, where each particle appears to be interfering with itself along the journey. The pattern revealed by many such particles shows interference, a purely quantum phenomenon.
- If you do measure which slit each particle goes through — particle 1 goes through slit 2, particle 2 goes through slit 2, particle 3 goes through slit 1, etc. — there is no interference pattern anymore. In fact, you simply get two “lumps” of particles, one each corresponding to the particles that went through each of the slits.
It’s almost as if everything exhibits wave-like behavior, with its probability spreading out over space and through time, unless an interaction forces it to be particle-like. But depending on which experiment you perform and how you perform it, quantum systems exhibit properties that are both wave-like and particle-like.
5.) The act of measuring a quantum system fundamentally changes the outcome of that system. According to the rules of quantum mechanics, a quantum object is allowed to exist in multiple states all at once. If you have an electron passing through a double slit, part of that electron must be passing through both slits, simultaneously, in order to produce the interference pattern. If you have an electron in a conduction band in a solid, its energy levels are quantized, but its possible positions are continuous. Same story, believe it or not, for an electron in an atom: we can know its energy level, but asking, “Where is the electron?” is something we can only answer probabilistically.
So you get an idea. You say, “Okay, I’m going to cause a quantum interaction somehow, either by colliding it with another quantum or passing it through a magnetic field or something like that,” and now you have a measurement. You know where the electron is at the moment of that collision, but here’s the kicker: by making that measurement, you have now changed the outcome of your system. You’ve pinned down the object’s position, you’ve added energy to it, and that causes a change in momentum. Measurements don’t just “determine” a quantum state, but create an irreversible change in the quantum state of the system itself.

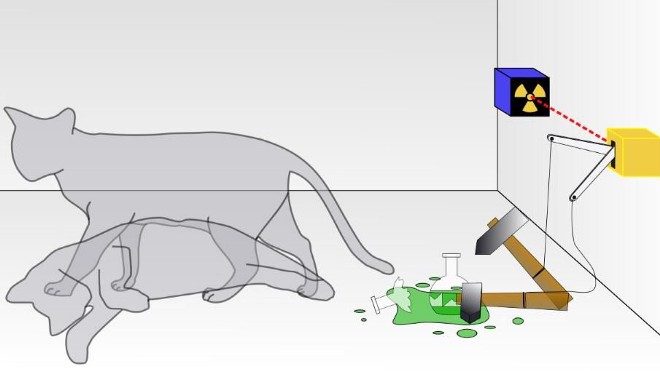
6.) Entanglement can be measured, but superpositions cannot. Here’s a puzzling feature of the quantum universe: you can have a system that’s simultaneously in more than one state at once. Schrodinger’s cat can be alive and dead at once; two water waves colliding at your location can cause you to either rise or fall; a quantum bit of information isn’t just a 0 or a 1, but rather can be some percentage “0” and some percentage “1” at the same time. However, there’s no way to measure a superposition; when you make a measurement, you only get one state out per measurement. Open the box: the cat is dead. Observe the object in the water: it will rise or fall. Measure your quantum bit: get a 0 or a 1, never both.
But whereas superposition is different effects or particles or quantum states all superimposed atop one another, entanglement is different: it’s a correlation between two or more different parts of the same system. Entanglement can extend to regions both within and outside of one another’s light-cones, and basically states that properties are correlated between two distinct particles. If I have two entangled photons, and I wanted to guess the “spin” of each one, I’d have 50/50 odds. But if I measured the spin of one, I would know the other’s spin to more like 75/25 odds: much better than 50/50. There isn’t any information getting exchanged faster than light, but beating 50/50 odds in a set of measurements is a surefire way to show that quantum entanglement is real, and affects the information content of the universe.
7.) There are many ways to “interpret” quantum physics, but our interpretations are not reality. This is, at least in my opinion, the trickiest part of the whole endeavor. It’s one thing to be able to write down equations that describe the universe and agree with experiments. It’s quite another thing to accurately describe just exactly what’s happening in a measurement-independent way.
Can you?
I would argue that this is a fool’s errand. Physics is, at its core, about what you can predict, observe, and measure in this universe. Yet when you make a measurement, what is it that’s occurring? And what does that mean about reality? Is reality:
- a series of quantum wavefunctions that instantaneously “collapse” upon making a measurement?
- an infinite ensemble of quantum waves, where measurement “selects” one of those ensemble members?
- a superposition of forward-moving and backward-moving potentials that meet up, now, in some sort of “quantum handshake?”
- an infinite number of possible worlds, where each world corresponds to one outcome, and yet our universe will only ever walk down one of those paths?
If you believe this line of thought is useful, you’ll answer, “Who knows; let’s try to find out.” But if you’re like me, you’ll think this line of thought offers no knowledge and is a dead end. Unless you can find an experimental benefit of one interpretation over another — unless you can test them against each other in some sort of laboratory setting — all you’re doing in choosing an interpretation is presenting your own human biases. If it isn’t the evidence doing the deciding, it’s very hard to argue that there’s any scientific merit to your endeavor at all.
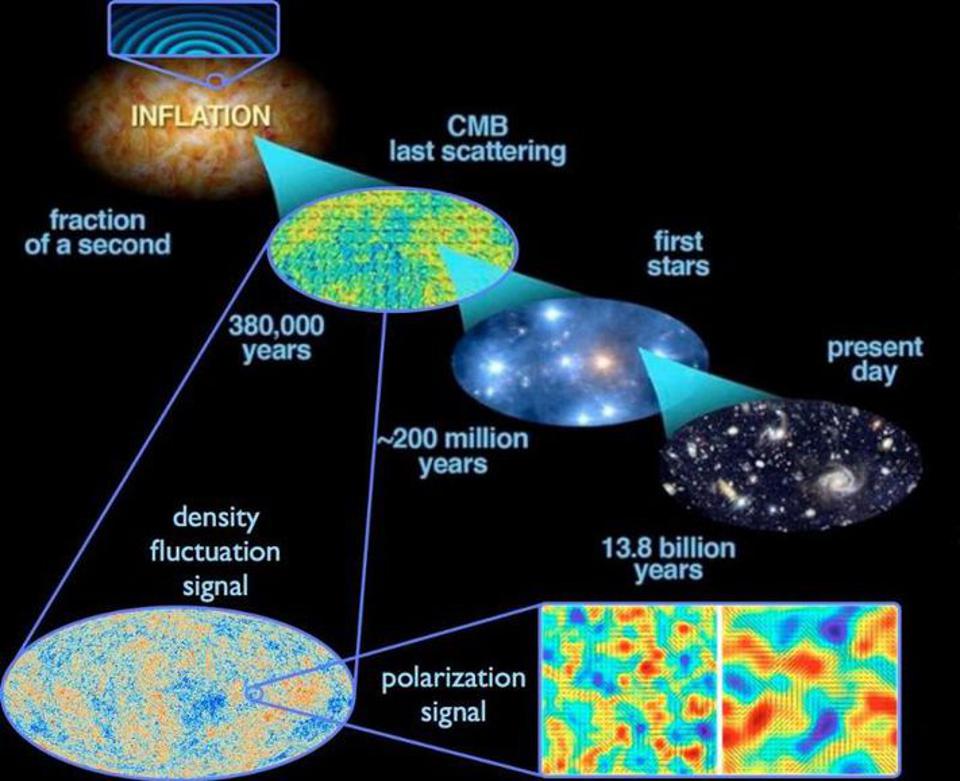
Credit: E. Siegel; ESA/Planck and the DOE/NASA/NSF Interagency Task Force on CMB research
If you were to only teach someone the classical laws of physics that we thought governed the universe as recently as the end of the 19th century, they would be utterly astounded by the implications of quantum mechanics. There is no such thing as a “true reality” that’s independent of the observer; in fact, the very act of making a measurement alters your system irrevocably. Additionally, nature itself is inherently uncertain, with quantum fluctuations being responsible for everything from the radioactive decay of atoms to the initial seeds of structure that allow the universe to grow up and form stars, galaxies, and eventually, human beings.
The quantum nature of the universe is written on the face of every object that now exists within it. And yet, it teaches us a humbling point of view: that unless we make a measurement that reveals or determines a specific quantum property of our reality, that property will remain indeterminate until such a time arises. If you take a course on quantum mechanics at the college level, you’ll likely learn how to calculate probability distributions of possible outcomes, but it’s only by making a measurement that you determine which specific outcome occurs in your reality. As unintuitive as quantum mechanics is, experiment after experiment continues to prove it correct. While many still dream of a completely predictable universe, quantum mechanics, not our ideological preferences, most accurately describes the reality we all inhabit.
This article was first published as an Ask Ethan column in October of 2021. It was updated in November of 2024.