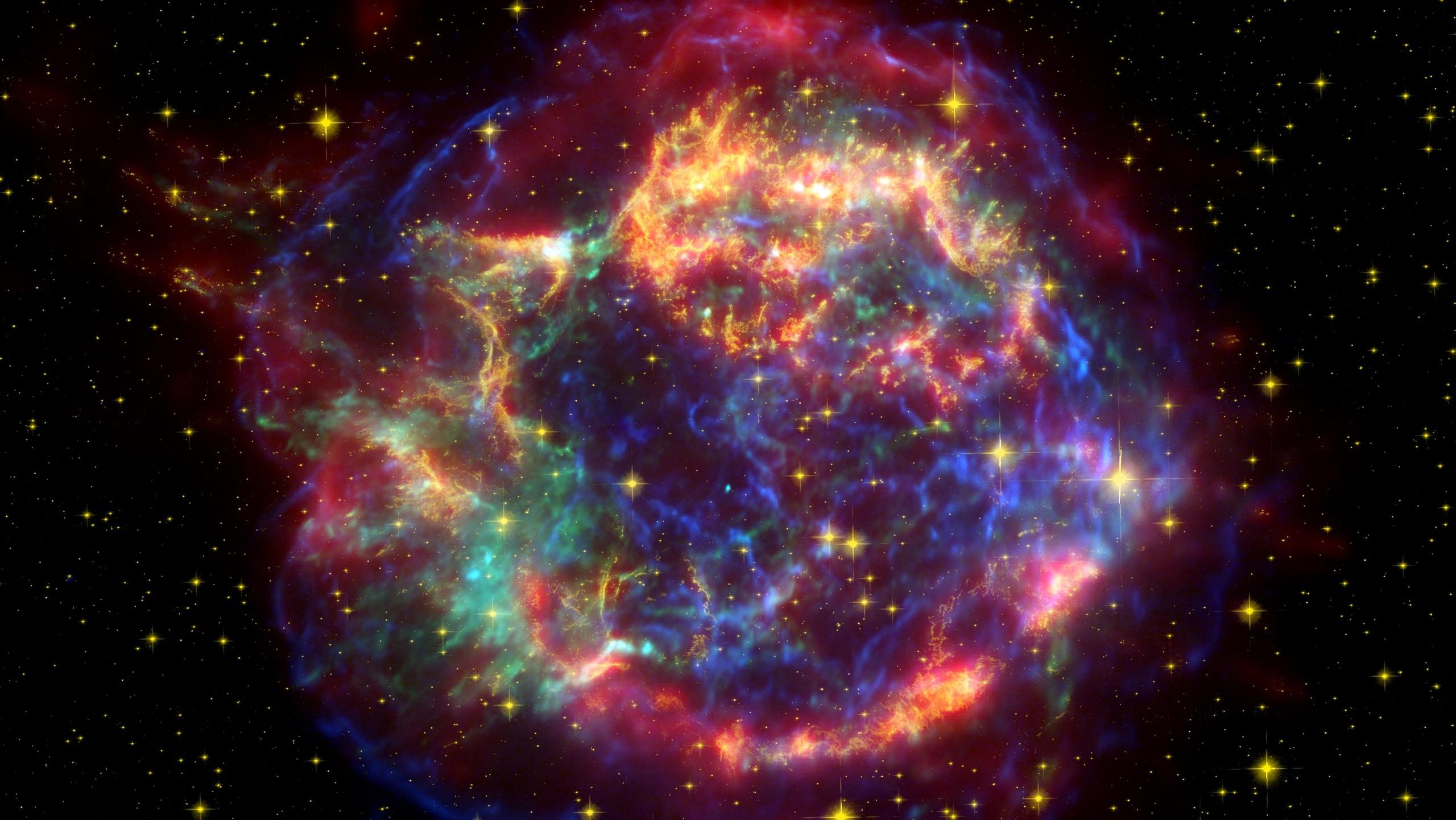
Ask Ethan: Did The Universe Have Zero Entropy At The Big Bang?

Entropy always increases, but that doesn’t mean it was zero to start with.
One of the most inviolable laws in the Universe is the second law of thermodynamics: that in any physical system, where nothing is exchanged with the outside environment, entropy always increases. This is true not only of a closed system within our Universe, but of the entire Universe itself. If you look at the Universe today and compare it to the Universe at any earlier point in time, you’ll find that the entropy has always risen and continues to rise, with no exceptions, throughout all of our cosmic history. But what if we go all the way back to the earliest times of all: to the very first moments of the Big Bang? If entropy has always increased, does that mean that the Big Bang’s entropy was zero? That’s what Vratislav Houdek wants to know, asking:
“According to the second thermodynamic law the total entropy is always growing. Does it mean at the moment of big bang the entropy was minimal (zero?), [implying that] the universe was maximally organized?”
The answer, perhaps surprisingly, is no. The Universe not only wasn’t maximally organized, but had quite a large entropy even in the earliest stages of the hot Big Bang. Moreover, “organized” isn’t quite a sound way to think about it, even though we use “disorder” as an offhand way to describe entropy. Let’s unpack what it all means.

When we think about the Universe in the earliest stages of the hot Big Bang, we’re imagining all the matter and radiation that we have today — currently spread out across a sphere some ~92 billion light-years in diameter — packed into a volume about the size of a soccer ball. It’s incredibly hot and dense, with some 10⁹⁰ particles, antiparticles, and quanta of radiation all possessing enormous energies billions of times what even the Large Hadron Collider at CERN can achieve. This includes:
- all the matter particles of the Standard Model,
- all their antimatter counterparts,
- gluons,
- neutrinos,
- photons,
- whatever’s responsible for dark matter,
- plus any exotic species of particles that may have existed,
all packed into a tiny volume with enormous kinetic energies. This hot, dense, expanding, and uniform to within 1 part in ~30,000 state would grow into the observable Universe we inhabit today over the next 13.8 billion years. Thinking about what we began with, however, it sure does seem like a disordered, very high-entropy state.
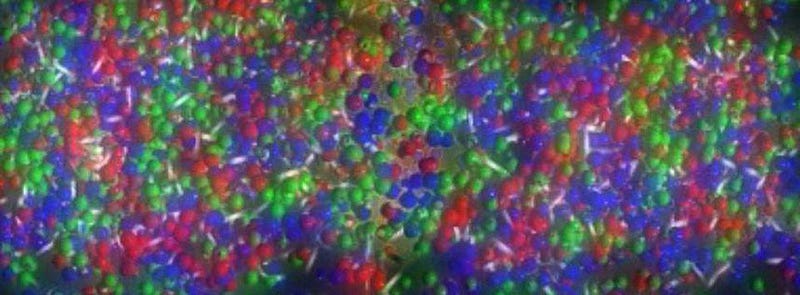
But what does entropy actually mean? We commonly talk about it as though it’s a measure of disorder: a broken egg on the floor has more entropy than an unbroken egg on the countertop; a cold dollop of cream and a hot cup of coffee have less entropy than the well-mixed combination of the two; a chaotic pile of clothes has a higher entropy than a neat set of dresser drawers with all the clothes folded and put away in an organized fashion. While these examples all correctly identify the higher-entropy versus the lower-entropy state, it isn’t precisely “order” or “disorder” that allows us to quantify entropy.
Instead, what we should be thinking about is — for all the particles, antiparticles, etc., that are present in the system — what the quantum state of each particle is, or what quantum states are allowed, given the energies and energy distributions at play. What entropy actually measures, rather than some nebulous characteristic like disorder, is this:
the number of possible arrangements of the quantum state of your entire system.
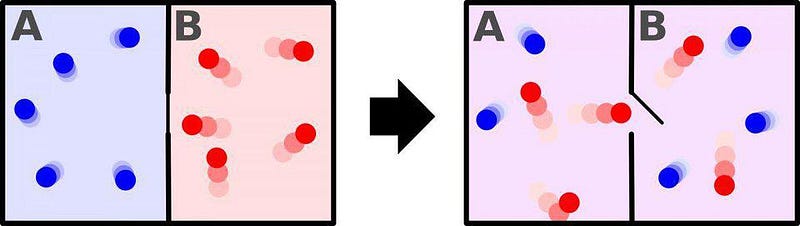
Consider the two systems above, for example. On the left, a box with a divider in the middle has cold gas on one side and hot gas on the other; on the right, the divider is opened and the entire box has gas of the same temperature. Which system has more entropy? The well-mixed one on the right, because there are more ways to arrange (or swap) the quantum states when all the particles have the same properties than when half have one set of properties and half have another, distinct set of properties.
When the Universe was extremely young, it had a certain number of particles in it, with a specific energy distribution to them. Almost all of the entropy, in these early stages, was due to radiation; if we calculate it, then we find that the total entropy was around S = 10⁸⁸ k_B, where k_B is Boltzmann’s constant. But every time an energy-emitting reaction occurs, such as:
- forming a neutral atom,
- fusing a light atomic nucleus into a heavier one,
- gravitationally collapsing a cloud of gas into a planet or star,
- or creating a black hole,
you increase the overall entropy of your system.

Today, the largest contributor to our Universe’s entropy is black holes, with today’s entropy reaching a value that’s about a quadrillion times as large as it was in the earliest stages of the Big Bang: S = 10¹⁰³ k_B. For a black hole, the entropy is proportional to the black hole’s surface area, which is larger for heavier-mass black holes. The Milky Way’s supermassive black hole, all on its own, has an entropy of approximately S = 10⁹¹ k_B, or around a factor of 1000 more than the entire Universe in the early stages of the hot Big Bang.
Over time, as the cosmic clock continues to tick by, we’ll form more and more black holes, while the heaviest black holes will gain mass. About 10²⁰ years from now, entropy will reach its maximum, as perhaps up to 1% of the mass of the Universe will form black holes, giving us an entropy somewhere in the range of S = 10¹¹⁹ k_B to S = 10¹²¹ k_B, an entropy that will (likely) only be conserved, not created or destroyed, as these black holes eventually decay via Hawking radiation.

But this is only for the observable Universe, which expands tremendously over time. If we were to compare the entropy density instead — or the entropy of the observable Universe divided by the volume of the observable Universe — that tells a very different story.
A soccer ball, with a radius of around 0.1 meters, has a volume of around 0.004 cubic meters, which means the very early Universe had an entropy density of a little over 10⁹⁰ k_B/m³, which is enormous. For comparison, the Milky Way’s central black hole, on its own, occupies a volume of around 10⁴⁰ m³, so its entropy density is only about 10⁵¹ k_B/m³, which is still extremely large, but much, much smaller than the entropy density of the early Universe.
In fact, if we look at the Universe today, even though the overall entropy is enormous, the fact that the volume is so large drives the entropy density to a relatively small number: about ~10²⁷ k_B/m³ to 10²⁸ k_B/m³.

Still, there’s a difference of about 15–16 orders of magnitude for the entropy in the early Universe, at the earliest moments of the hot Big Bang, as compared to the entropy today. Over the cosmic history of the Universe, even though the expansion has diluted the entropy density — or the amount of entropy-per-unit-volume — the total entropy has increased dramatically.
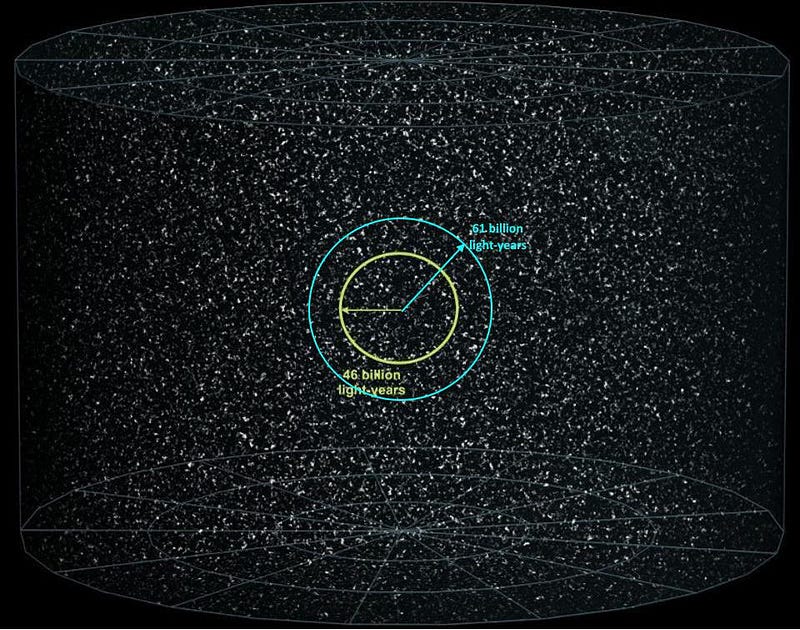
However, there’s a difference between the observable Universe, which we can see and measure today, and the unobservable Universe, which remains largely unknown to us. Although we can presently see for 46 billion light-years in all directions, and as time goes on, even more of the expanding Universe will eventually be revealed to us, we only have a lower limit on the size of the Universe beyond the part we can observe. For all we know, space might truly be infinite beyond that.
But it’s important to remember that the Big Bang, although it’s the origin of our Universe as we know it, isn’t the very first thing of all that we can sensibly talk about. As far as we can tell, the Big Bang wasn’t the very beginning, but rather describes a set of conditions — hot, dense, almost perfectly uniform, expanding, filled with matter, antimatter, and radiation, etc. — that existed at some early time. In order to set up the Big Bang, however, the best evidence we have points to another state preceding the Big Bang: cosmic inflation.
According to inflation, before the Big Bang, the Universe was filled with a dark energy-like form of energy: energy inherent to a field or the fabric of space itself, rather than particles, antiparticles or radiation. As the Universe expanded, it did so exponentially: relentlessly, rather than at an ever-decreasing rate determined by the falling density of matter and radiation. During this time, for however long it went on, with every ~10^-32 s or so that passed, a region the size of the Planck length, the smallest scale at which the laws of physics don’t break down, gets stretched to the size of today’s presently visible Universe.
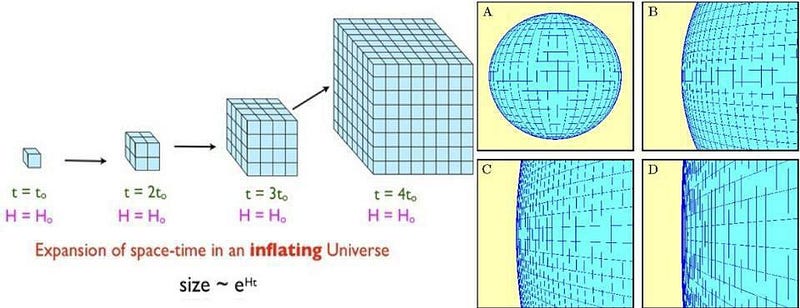
During inflation, the entropy of our Universe must have been much, much lower: around 10¹⁵ k_B for a volume equivalent to the size of our observable Universe as the start of the hot Big Bang. (You can calculate it for yourself.) But what’s important is this: the entropy of the Universe doesn’t particularly change all that much; it simply gets diluted. The entropy density changes dramatically, but whatever pre-existing entropy was present in the Universe prior to inflation still remains (and can even increase), but gets stretched across larger and larger volumes.
This is vital to understanding what happens in our Universe. We don’t need some miraculously low-entropy state to occur to begin our Universe or to begin the process of inflation. All we need is for inflation to arise in some part of the Universe and for that space to begin inflating. In short order — after no more than a tiny fraction of a second — no matter how much entropy there initially was, that entropy is now spread out over a much larger volume. Entropy may always be increasing, but the entropy density, or the amount of entropy contained in the volume that will someday become our entire observable Universe, drops to this extremely low value: about 10 nanojoules-per-Kelvin, spread out over the volume of a soccer ball.
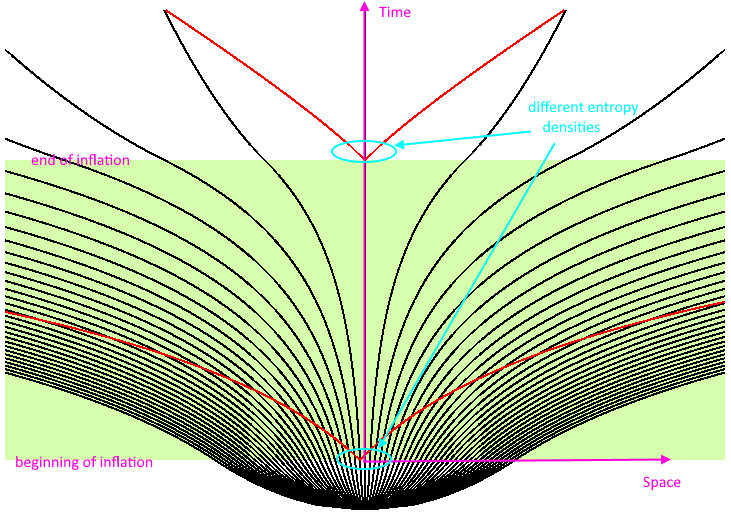
When inflation ends, that field energy gets converted into matter, antimatter and radiation: that hot, dense, almost uniform, and expanding-but-cooling Universe. Converting that field energy into particles causes the entropy within our observable Universe to rise dramatically: by about 73 orders of magnitude. Over the next 13.8 billion years, as our Universe expanded, cooled, fused, gravitated, formed atoms and stars and galaxies and black holes and planets and humans, our entropy “only” rose by 15 or 16 orders of magnitude.
What has occurred and what will occur over the entire history of the Universe is peanuts compared to the biggest entropy growth to ever happen: the end of inflation and the beginning of the hot Big Bang. Yet even during that inflationary state with alarmingly low entropy, we still never saw the entropy of the Universe decrease; it was only the entropy density that went down as the volume of the Universe increased exponentially. In the far future, when the Universe expands to about 10 billion times its present radius, the entropy density will once again be as small as it was during the inflationary epoch.
Although our entropy will keep on increasing, the entropy density will never be as large as it was at the start of the hot Big Bang, some 13.8 billion years ago.
Send in your Ask Ethan questions to startswithabang at gmail dot com!
Starts With A Bang is written by Ethan Siegel, Ph.D., author of Beyond The Galaxy, and Treknology: The Science of Star Trek from Tricorders to Warp Drive.