Ask Ethan: Can Normal Stars Make Elements Heavier (And Less Stable) Than Iron?

It isn’t just supernovae or neutron star collisions that make the heaviest elements. The physics might surprise you!
“Comrades, this man has a nice smile, but he’s got iron teeth.”
–Andrei A. Gromyko
There are some 90+ elements of the periodic table that occur naturally in the Universe, but of them all, iron is the most stable. If you fuse lighter elements together to get closer to iron, you gain energy; the same is true if you split heavier elements apart. Iron represents the most stable configuration of protons and neutrons, combined, of any atomic nucleus yet discovered. At only element 26, however, it represents the end-of-the-line for most fusion reactions in even the most massive stars. Or does it? That’s what James Beall wants to know:
Iron has been called stuff like solar fusion ash that collects inside stars, as the last of the elements that fuse w/o consuming more energy than the fusion creates. I have read about the r-process and others that lead to heavier elements in novas and supernovas. My Q is if any elements heavier than iron fuse anyway in normal stars, even if it does consume more energy then it generates.
The answer, as you might expect, is a little complicated: you do make heavier elements than iron in normal stars, but only a very small amount comes from fusion.

All stars begin by fusing hydrogen into helium, from the tiny red dwarfs as little as 8% the mass of our Sun, up to the largest, most massive stars in the Universe that weigh in at hundreds of times the mass of our own. For about 75% of these stars, helium is the end of the line, but the more massive ones (like our Sun) will develop a red giant phase, where they fuse helium into carbon. But a very tiny percentage of stars — just over 0.1% — are among the most massive of all, and can initiate carbon fusion and beyond. These are the stars destined for supernovae, as they fuse carbon into oxygen, oxygen into silicon and sulphur, and then enter the final burning phase (silicon-burning) before going supernova.
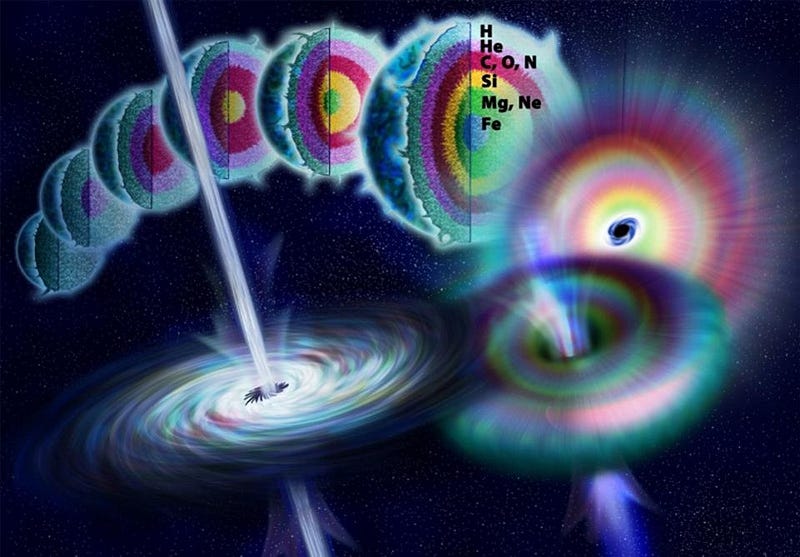
That’s the normal life-cycle of the most massive stars in the Universe, but “silicon-burning” doesn’t work by smashing two silicon nuclei together to build something heavier. Instead, it’s just a chain reaction of the addition of helium nuclei to a silicon nucleus, occurring at temperatures in excess of 3,000,000,000 K, or more than 200 times the temperature at the center of the Sun. The chain reaction proceeds as follows:
- silicon-28 plus helium-4 yields sulphur-32,
- sulphur-32 plus helium-4 yields argon-36,
- argon-36 plus helium-4 yields calcium-40,
- calcium-40 plus helium-4 yields titanium-44,
- titanium-44 plus helium-4 yields chromium-48,
- chromium-48 plus helium-4 yields iron-52,
- iron-52 plus helium-4 yields nickel-56, and
- nickel-56 plus helium-4 yields zinc-60.
You’ll notice there’s no iron-56 produced, and there are two reasons why.
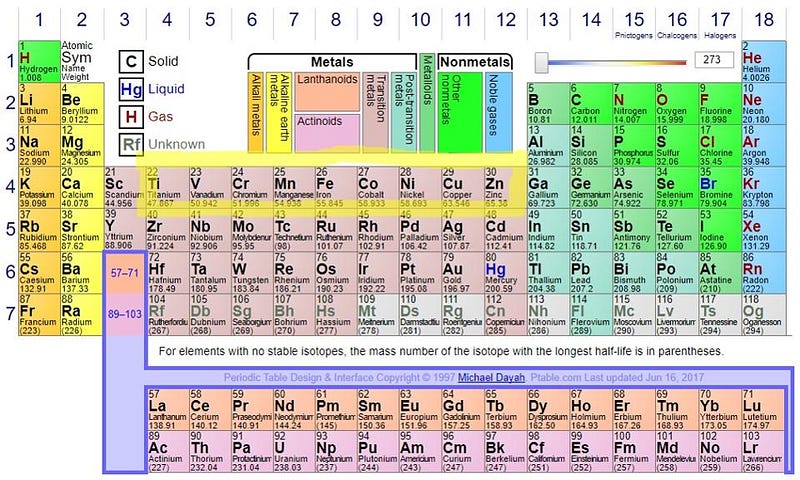
One is that, if we look at this portion of the periodic table, we can see that there are too few neutrons for the number of protons in these nuclei. Iron-52, for instance, is unstable; it emits a positron and decays to manganese-52, moving down the periodic table. (The manganese then emits another positron and decays to chromium-52, which is stable.) The nickel-56 is also unstable, decaying to cobalt-56, which then decays to iron-56, and is how we arrive at the periodic table’s most stable element. And zinc-60 decays first to copper-60, which then decays again to nickel-60. All of these end products are stable, so yes, these stars — even before going supernova — can produce cobalt, nickel, copper, and zinc, all of which are heavier than iron.
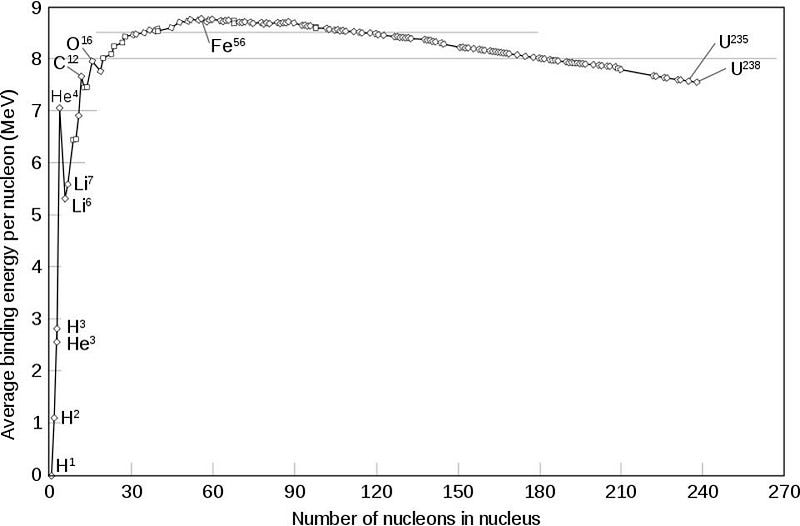
If this isn’t energetically favorable, though, how is this possible? I want you to look at the chart above, which details the binding energy per nucleon in each of the atomic nuclei. I want you to notice how flat the chart is near iron-56; many elements on either side have almost the exact same binding energy per nucleon. Now look all the way over on the left side to helium-4. What do you notice?
Helium-4 is not as tightly bound as any of the nuclei around iron-56. So even though, for example, zinc-60 might have less binding energy per nucleon than nickel-56, it still has more binding energy per nucleon than nickel-56 combined with helium-4. Overall, the net reaction is positive. What we wind up with, therefore, in the last moments before a supernova, is a mix of elements all the way up to zinc: a full four elements heavier than iron.

You might wonder about even heavier elements, then. Would it be possible to, say, add another helium-4 nucleus to zinc-60, producing germanium-64? In trace amounts, probably, but not in any significant quantities. The simple reason? In part, it’s that the energy difference is now almost exactly zero between the two states. But more significantly, you run out of time. For an extremely massive star, the lifetime of the various stages are approximately:
- Hydrogen fusion: millions of years
- Helium fusion: hundreds of thousands of years
- Carbon fusion: hundreds to one thousand of years
- Oxygen fusion: months to one year
- Silicon fusion: hours to one-or-two days.
In other words, that final stage — the one that produces iron and the iron-like elements — doesn’t last long enough to go beyond that.
But if you’re willing to consider what takes place inside a massive star that already has iron and iron-like elements, you can build your way all the way up to lead and bismuth. You see, once you’ve had supernovae in the Universe, you have significant amounts of iron, cobalt, nickel, etc., and these heavy elements wind up in new generations of stars that form. In stars that are between 60–1000% as massive than the Sun (but not usually massive enough for supernovae), you can fuse carbon-13 with helium-4, you can produce oxygen-16 and a free neutron, while stars that will go supernova will fuse neon-22 with helium-4, producing magnesium-25 and a free neutron. Both of these processes can build up heavier and heavier elements, reaching all the way up to lead, bismuth, and even (temporarily) polonium.
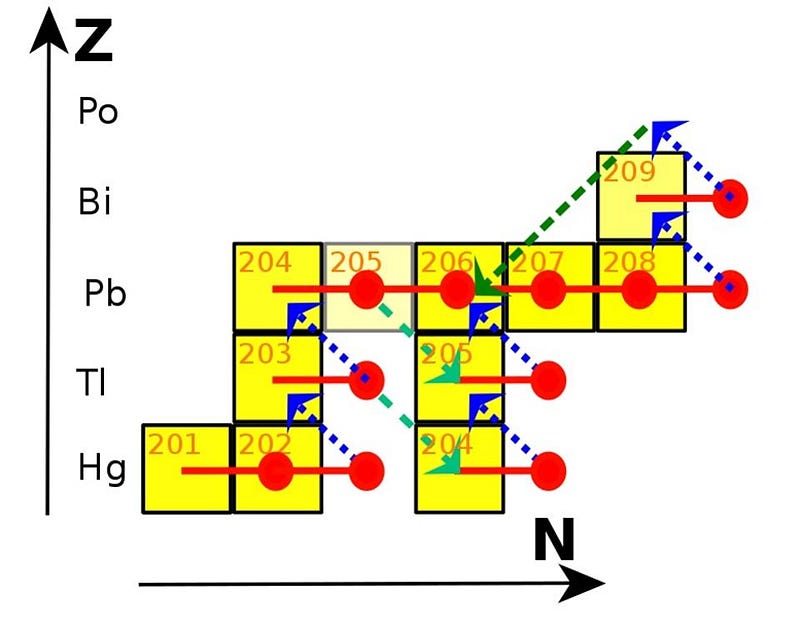
Perhaps ironically, it’s the higher-mass stars that produce large amounts of the lighter elements (up to rubidium and strontium or so: elements 37 and 38), while the lower-mass (non-supernova) stars will take you the rest of the way up to lead and bismuth. It isn’t technically a fusion reaction; it’s neutron capture, but it’s how you build up the heavier and heavier elements. The biggest reason why the lower-mass stars can get you to such great heights, metaphorically?
It’s time.
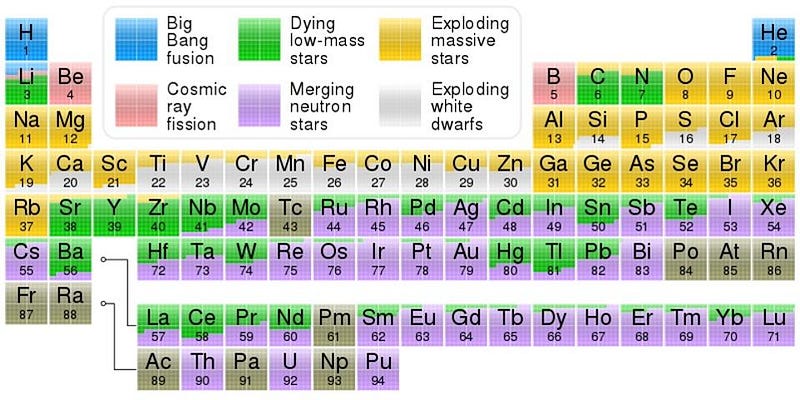
The lower-mass stars remain in this neutron-producing state for tens or even hundreds of thousands of years, while the stars destined for supernovae produce neutrons for only hundreds of years, or even fewer. The energy concerns are a really big deal when it comes to fusion; even at temperatures of billions of degrees, reactions still proceed in the direction that’s more energetically favorable. But precious time is the biggest constraint for building up heavier and heavier elements. Incredibly, with the right combination of neutron capture and nuclear fusion, about half of all the elements beyond iron are produced inside stars, without supernovae or merging neutron stars at all.
Send in your Ask Ethan questions to startswithabang at gmail dot com!
Ethan Siegel is the author of Beyond the Galaxy and Treknology. You can pre-order his third book, currently in development: the Encyclopaedia Cosmologica.