Ask Ethan: How Can Worlds That Never Get Above Freezing Have Liquid Water?

How three factors come together to unfreeze the ice, and unlock the potential for extraterrestrial life.
“Day after day, day after day,
We stuck, nor breath nor motion;
As idle as a painted ship
Upon a painted ocean.
Water, water, every where,
And all the boards did shrink;
Water, water, every where,
Nor any drop to drink.” –Samuel Taylor Coleridge
The Solar System has proved a surprising place, and perhaps one of the biggest surprises is that Earth isn’t the only world with liquid water on its surface. Sure, there’s a tiny bit that temporarily exists on Mars, but worlds like Jupiter’s moon Europa, Saturn’s Enceladus, and even ultra-distant Pluto all harbor enormous subsurface oceans, with some of these worlds having even more water than Earth. Yet unlike Earth or even Mars, these worlds are so distant from the Sun and so cold that the warmest surface temperature never even approaches water’s melting point. So how do they have liquid water? That’s what Gary Lapidus wants to know:
I was reading about Saturn’s moon Enceladus, and how scientists believe it has liquid water oceans beneath its water-ice crust. And yet I also read that the warmest surface temperatures are -90 celsius. How can this moon have liquid water? […] At such cold temperatures and low pressures it seems Enceladus can have water ice and water gas but not liquid. What am I missing?
Let’s start with water as we know it here on Earth to find out.

On Earth, water can exist in three phases: solid, liquid, and gas, all depending on what temperature it exists at. Below 32° F (0° C), water freezes into ice; above that but below 212° F (100° C), water is liquid; above 212° F (100° C), it exists as gaseous water vapor. This is the way you learn water works as a kid, and it’s right, mostly. But there are a few conditions that can make water behave very differently. For example, if you live in a high-altitude location, like Bogotá, Colombia, Quito, Ecuador, or El Alto, Bolivia, all of which have over a million residents, your water boils at a far lower temperature. (Large sections along the Rocky Mountain range in the USA are at substantial, albeit lower, elevations as well.)
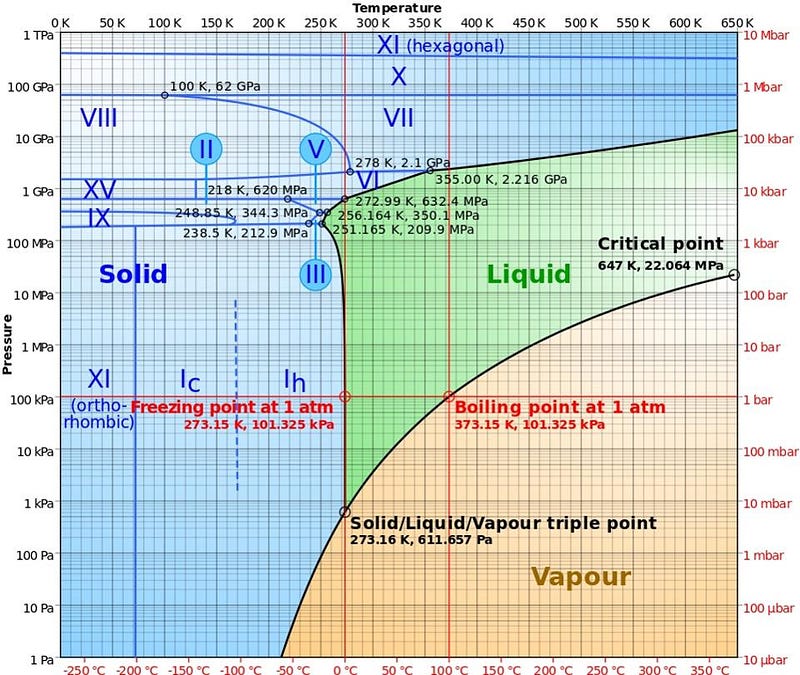
This is because the pressure surrounding you affects both the boiling points and freezing points of water. In the depths of space, with no atmosphere, liquid water is impossible; water can only exist in either the solid or gaseous phases. But here on Earth, water boils at lower temperatures at lower pressures, while frozen water, if enough pressure is applied, will actually melt and become liquid. This latter point often surprises people, until they’re asked to think about ice skates. If you head out onto the ice without skates, it’s extremely slippery and very difficult to control your motion or gain traction; your shoes slide over the frozen surface of the ice. But with skates, all the force from your weight is concentrated onto a single blade, increasing the pressure on the ice many times over, causing it to temporarily liquefy into a watery state.

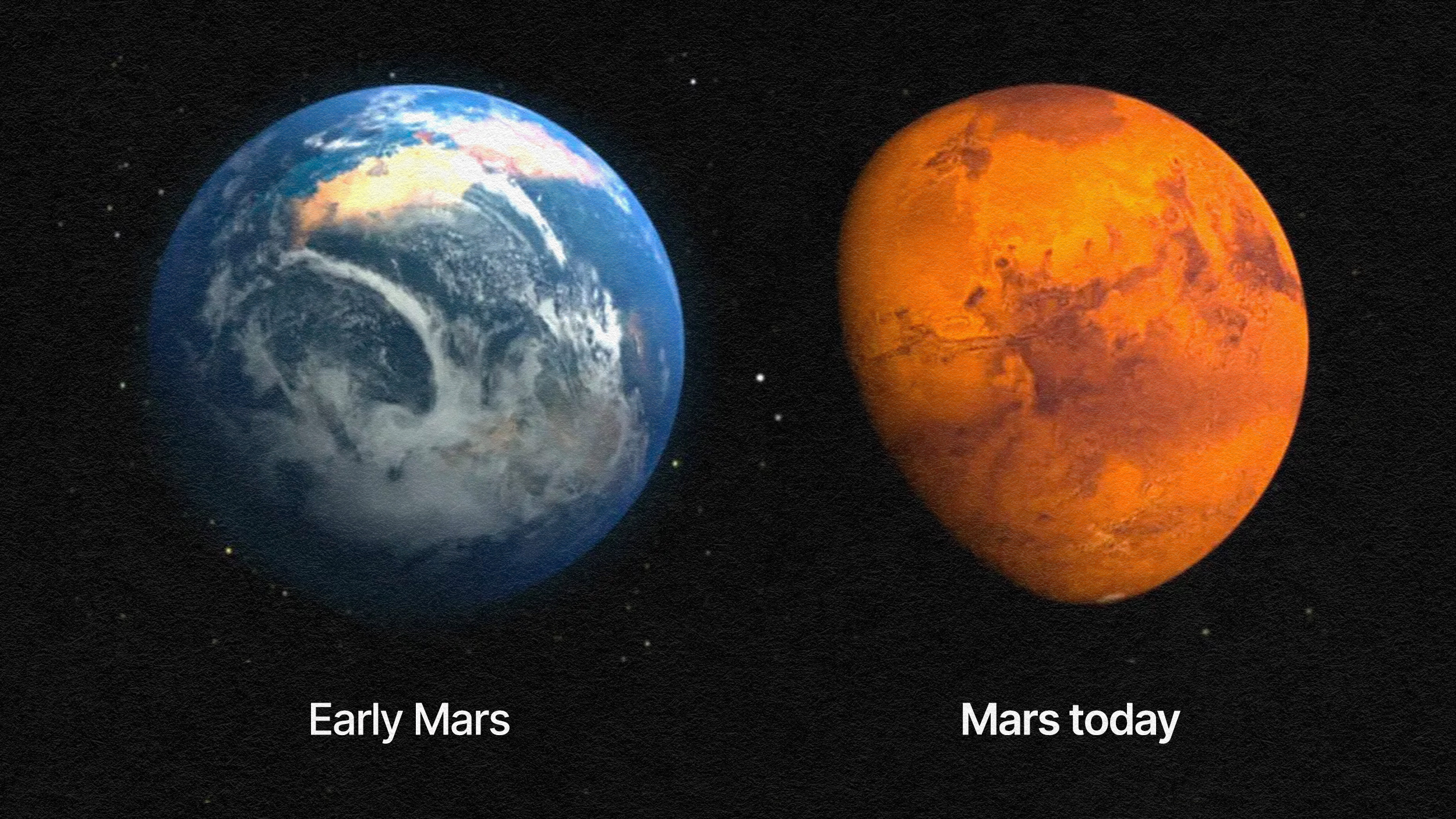
There’s also something else worth considering when it comes to water: the freezing point of water changes depending on what’s dissolved in it. If you’ve ever put a bottle of vodka in the freezer, you’ll know that water mixed with 40% alcohol doesn’t freeze at the same temperature that pure water does, but at a much lower one. Our ocean, too, with its dissolved salts, also experiences a lower freezing point than pure water alone: 28° F (-2° C) at the approximately 4% salinity content. So you can have colder temperatures than water’s normal freezing point and still have liquid water, depending on what else is in it. This is one of the most striking features of Mars, where pure liquid water ought to be unable to exist.

At the pressures and temperatures present on the surface of Mars, liquid water should be a physical impossibility. But thanks to the high salt content of some martian soils, when water condenses on the surface it can exist in a liquid phase. The flowing channels down the slopes of crater walls — known as recurring slope lineae — were the first direct evidence of liquid water on the surface of another world beyond Earth.
Yet if we look farther out in the Solar System, to worlds like Europa, Enceladus, or even as far away as Pluto, there is no surface water to be found.

A close examination of these worlds shows only ice. Yes, it is water-ice, which is promising, but the temperatures of these worlds, at many times the Earth-Sun distance, means that temperatures not only never approach the 32° F (0° C) temperatures needed to have liquid water on Earth’s surface, but that they never approach the temperatures necessary to have liquid water at any allowable pressure. Still, if we were to go beneath the icy surfaces of these worlds, we would get a lot closer, because there is a tremendous pressure increase underneath all that ice.

It takes the entire 60 miles (100 kilometers) of atmosphere above us to create the atmospheric pressure we feel at sea level, yet it only takes another 34 feet (10 meters) under water to double that pressure. On another world, ice can easily be tens of thousands of feet thick, generating tremendous pressures that bring us quite close to the liquid phase. Even with the briny deposits present in the ice, liquid water still won’t be the result without one additional factor: a heat source. Thankfully, each of these worlds has a heat source: a nearby, massive, orbiting companion.
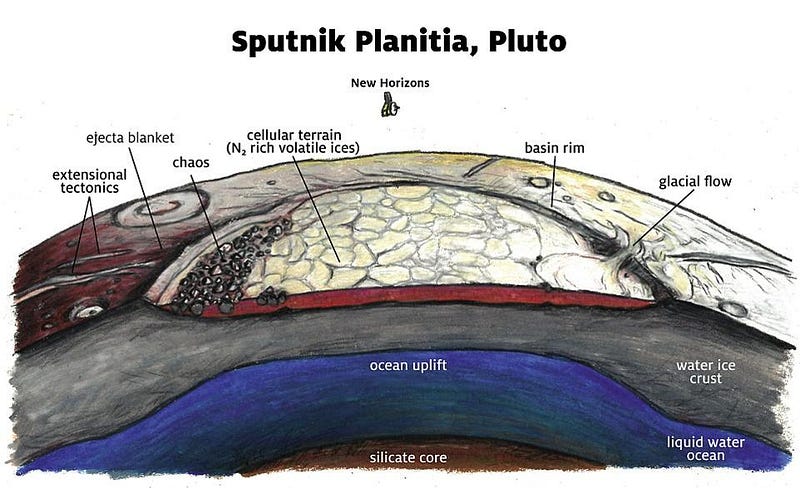
Europa has Jupiter; Enceladus has Saturn; Pluto has its nearby moon Charon. All three, with their combinations of large masses and relatively close proximities, exert very large tidal forces on these worlds. These forces don’t just cause slight deformations in their outer layers, but stretch, compress, and shear the interiors of these worlds, causing them to heat up. If you work out the amount of tidal heating present, and add in the effects of the pressure from the ice above and the salts beneath the outer, icy layers, you’ve finally arrived at what you’ve been seeking: a liquid ocean beneath the surface of ice.

Europa exhibits enormous cracks in its surface, evidence of where the ice has broken and water has emerged in the past. Enceladus’ subsurface ocean is the most spectacular, with giant eruptions of liquid water spewing forth from the surface and extending hundreds of kilometers into space. The plumes of Enceladus are so dramatic that they’ve even become responsible for the creation of one of the rings of Saturn: the E-ring. Finally, Pluto, in perhaps the greatest surprise of all, was determined to have a subsurface liquid ocean of water beneath its frozen surface. And where there’s water, heat, and dissolved chemicals, it’s conceivable — albeit very speculative — that there may be something better than water beneath the surfaces of these worlds, too.
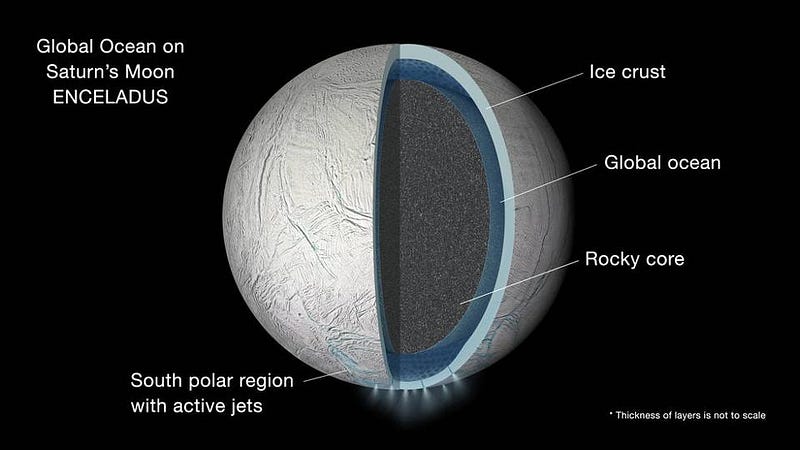
Could there possibly be life on a world where sunlight never penetrates down to the liquid ocean that might house that life? It’s possible, and of these three worlds, it’s conceivable that Enceladus might be the first one to be put to the test. Its geysers mean it’s eminently plausible for sunlight to catalyze some of the biochemical molecules that might give rise to life, before falling back down onto the icy moon’s surface. Over long enough time periods, enough ice can accumulate atop them that the pressure will cause the ice to become liquid again, perhaps creating a long-term, life-giving cycle on this world. To find out, we wouldn’t have to dig or crash a probe onto this moon, but simply to fly a mission through one of Enceladus’ geysers and collect a sample. Could life beyond Earth be so easily within reach in the Solar System? Perhaps, if we’re lucky, someday we’ll all get to find out.
Send in your Ask Ethan submissions to startswithabang at gmail dot com!
Ethan Siegel is the author of Beyond the Galaxy and Treknology. You can pre-order his third book, currently in development: the Encyclopaedia Cosmologica.