Throwback Thursday: The Physics of Volcanic Lightning
It’s one of the most beautiful (and terrifying) sights in the world. But what causes it?
“If you are caught on a golf course during a storm and are afraid of lightning, hold up a 1-iron. Not even God can hit a 1-iron.” –Lee Trevino
Here on Earth, nature puts on all sorts of destructive displays of power. From hurricanes to tornadoes to avalanches, tsunamis and earthquakes, natural phenomena are routinely capable of laying waste to entire cities. But perhaps no destructive force is more fascinating than the volcano.

From deep within the Earth’s mantle, liquid rock heated to thousands of degrees makes its way upwards to the crust, and in a few select weak points, can erupt through to the surface. When this happens, not only does lava emerge, but is often accompanied by large amounts of soot and ash.
And occasionally, if the recipe is just right, lightning as well.

There’s an awful lot we don’t even understand about normal lightning that occurs in thunderstorms, much less in volcanic lightning. Yet, just because we don’t know everything about it doesn’t mean we can’t speak intelligently about this frighteningly beautiful phenomenon, and talk about the physics we do know.

So when is it that volcanic lightning is most likely to occur, and — to the best of our understanding — what causes it?
First off, it appears to occur most frequently around volcanoes with large ash plumes, and has been exquisitely recorded around a number of recent volcanic eruptions, including Iceland’s Eyjafjallajökull, Japan’s Sakurajima, and Chile’s Puyehue and Chaiten volcanoes. But what you may not know is this phenomenon was not only captured during Mt. Vesuvius’ last eruption in 1944, but was accurately described nearly 2,000 years ago when it erupted in the year 79!

Each lightning strike is the exchange of some 10^20 electrons, or — because I want you to see it written out — 100,000,000,000,000,000,000 charged particles.
You may be used to atoms being neutral — with equal numbers of electrons as there are protons in their nuclei — but heat and friction make it surprisingly easy for atoms to gain or lose electrons, and become ions.
At the temperatures that volcanoes achieve, it’s energetically favorable for an atom to become ionized, where it either picks up or loses an electron (or two, or three). We certainly don’t need to go these extremes to find ions; something as simple as table salt (or rubbing wool socks against the carpet) is an example.
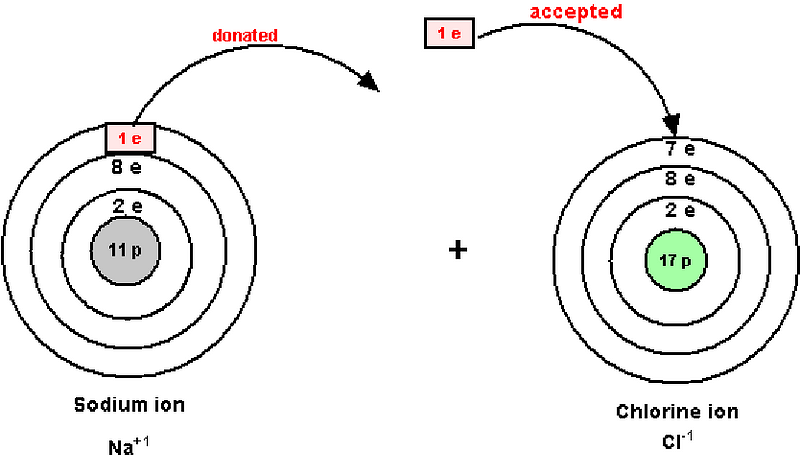
Now, if you can separate these ions from one another, you create a separation of charge, which creates a Voltage. When the Voltage (also known as the electric potential difference) between two regions becomes too great — even if air is the only thing between them — it spontaneously becomes conductive, and this rapid exchange of charge is what you see as a lightning strike!
All told, there have been more than 150 different eruptions over the past couple of centuries where volcanic lightning has been recorded.

So, with all this in mind, how does this happen? Let’s walk you through step-by-step.
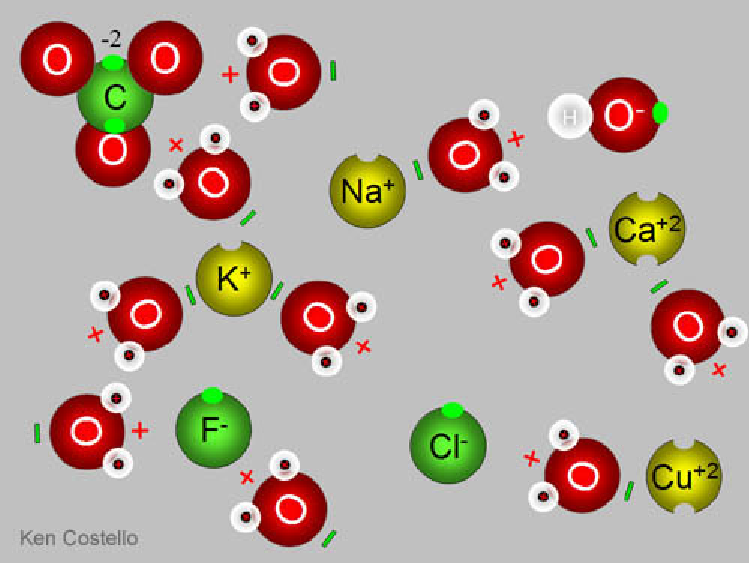
1.) Abundance of positive and negative ions. The combination of heat (around 1,500 Kelvin, for a typical volcano) and the diverse composition of what’s unearthed by a volcano ensures a significant minority of particles coming out are not neutral. Electrons can relatively easily be kicked off of some molecules and absorbed by others; for the individual ash particles that come out, many are positively charged ions and many are negatively charged ions.
In addition to their charges differing from one another, they also have different molecular (or atomic) weights from one another, as well as different physical sizes (or cross-sections). This is extremely important, because it allows for step 2.
2.) We’ve got to separate the negative charges from the positive ones. Neutral atoms have different physical sizes from one another, and charged atoms (and molecules) have that difference exaggerated even moreso. There are also significant mass differences between different atoms-and-molecules, which is important because giving the same amount of energy to a lighter particle means it winds up moving faster. And finally, there’s also a temperature gradient, where the particles just coming out have higher temperatures than the ones that have been in the atmosphere for some time.
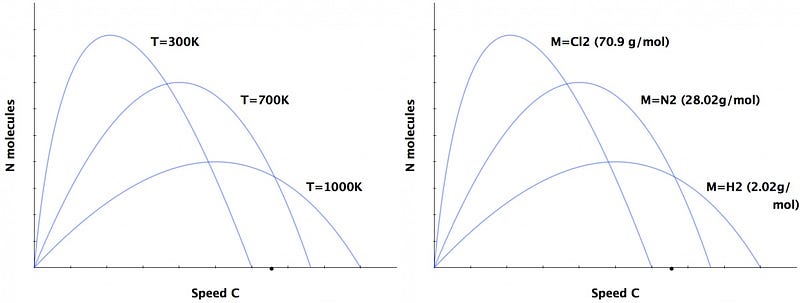
This combination of different temperatures and different masses give these ions different speeds from one another. And when you have a turbulent environment, smaller and lighter particles are typically transported greater distances more easily, making it easy for charges to separate by large distances.
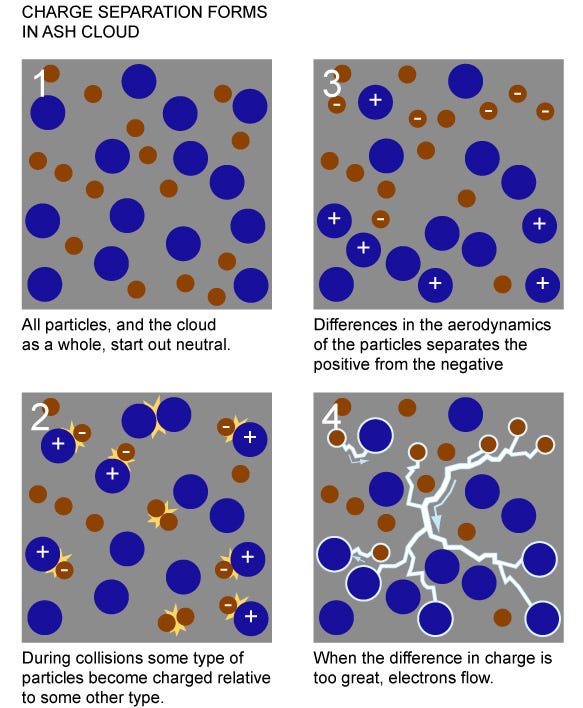
3.) When there’s a large enough voltage difference, you get an electrical discharge, which is a lightning strike! And that’s the general process behind how volcanic lightning works.
Combine these things together: different mass (and charge) ions moving at different average speeds with different cross sections in an environment with a temperature gradient, and there’s your recipe for a charge separation! Get one that’s large enough, and what does that give you?

Volcanic lightning!
There are still a number of details to be filled in as far as how this occurs in volcanic eruptions, why it sometimes occurs in the virtual absence of ash clouds, and why some volcanoes don’t appear to have it at all. But this basic picture is irrefutable, and has given us some spectacular sights for all the world to share in.

The more spectacular images — like these — tend to be time-lapse images, where many lightning strikes occurring over time periods of minutes or even hours are shown in the same frame.

And that’s the physics of volcanic lightning, along with some amazing images. Hope you enjoyed it!
Have a question, comment, or a desire to weigh in? Join us at the Starts With A Bang forum over on Scienceblogs, and drop us a line!