Breakthrough Tech: Scientists Use Swarms of Nanorobots to Precisely Target Cancer Cells
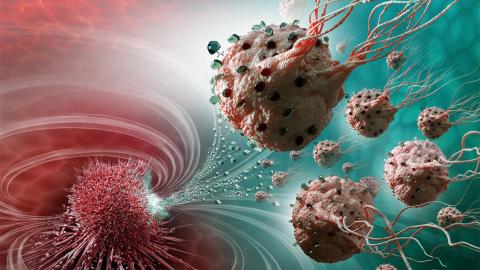
Nanotechnology has always been something more out of the future than the present. It’s very promising tech with a variety of exciting applications that haven’t yet come to our everyday lives. Now scientists from several universities announced a breakthrough in cancer research that may truly fulfill the promise of nano tech. They developed nanorobots that can navigate through the bloodstream to precisely deliver the drug to the cancerous cells.
Injecting the medication this way targets the tumor without jeopardizing other organs and tissues. One significant implication of this method is the potential reduction in the amount of harmful chemotherapy drugs that are given to a cancer patient.
The research was successfully carried out on mice by teams from Polytechnique Montréal, Université de Montréal and McGill University under the leadership of Professor Sylvain Martel, who is Canada’s Research Chair in Medical Nanorobotics and Director of the Polytechnique Montréal Nanorobotics Laboratory.
“These legions of nanorobotic agents were actually composed of more than 100 million flagellated bacteria – and therefore self-propelled – and loaded with drugs that moved by taking the most direct path between the drug’s injection point and the area of the body to cure,” explains Professor Martel.
The nanorobots are actually a strain of Magnetococcus marinus bacteria known as MC-1, each cell equipped with magnetic iron-oxide nanocrystals.
These bacteria enter a tumor, then detect oxygen-depleted tumor areas called hypoxic zones and inject the drug straight into them. These hypoxic zones are generally resistant to most therapies like radiotherapy.
The researchers managed to get the MC-1 nanocarriers right to the targeted areas, with up to 55% of MC-1 cells penetrating into the hypoxic areas of the tumors in mice.
“The drug’s propelling force was enough to travel efficiently and enter deep inside the tumors,” says Professor Martel.
How do the robots know where they need to go?
The new transportation systems is where the real breakthrough comes in. The bacteria used by the researchers has a type of compass made by synthesizing a chain of magnetic nanoparticles. The bacteria move in the direction of a computer-controlled magnetic field while utilizing a sensor measuring the concentration of oxygen in active tumor areas.
“This innovative use of nanotransporters will have an impact not only on creating more advanced engineering concepts and original intervention methods, but it also throws the door wide open to the synthesis of new vehicles for therapeutic, imaging and diagnostic agents,” Professor Martel elaborates.
“Chemotherapy, which is so toxic for the entire human body, could make use of these natural nanorobots to move drugs directly to the targeted area, eliminating the harmful side effects while also boosting its therapeutic effectiveness.”
Human trials for this technology have not been announced yet. The study’s co-author Nicole Beauchemin, professor of Biochemistry, Medicine and Oncology at McGill University and researcher at the Rosalind and Morris Goodman Cancer Research Centre, is optimistic for them to start:
“These results represent a novel therapeutic avenue for patients with hard-to-treat cancers, once the approach has been validated in human trials. The combination of multiple expertise from all team members has made this project possible; it represents for me one of the most exciting scientific projects I have ever tackled.”
You can read the paper “Magneto-aerotactic bacteria deliver drug-containing nanoliposomes to tumour hypoxic regions” in Nature Nanotechnology.