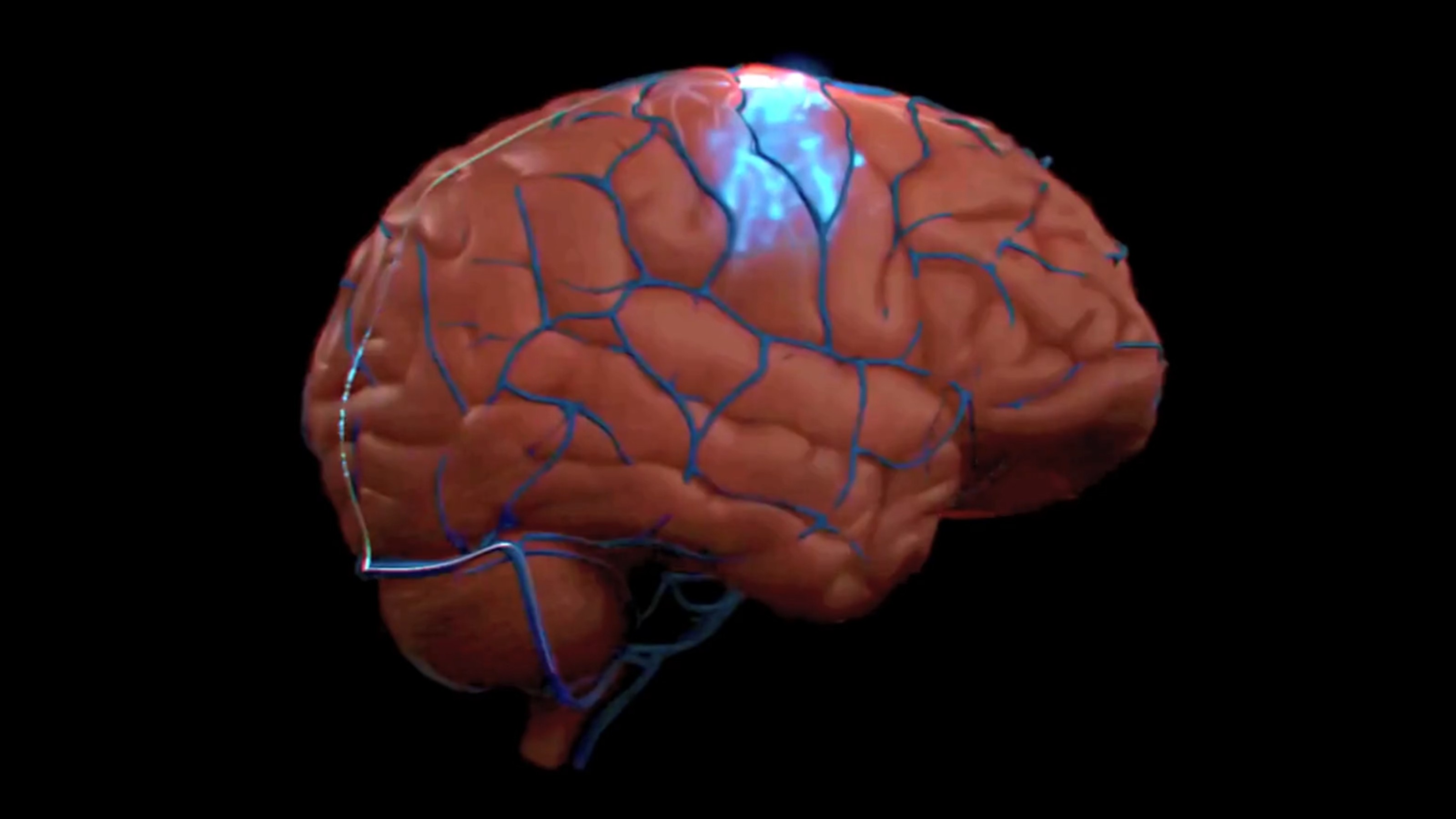
Amyloid plaques and neurofibrillary tangles inside the brain are the best explanation we have for how Alzheimer’s develops.
The Brain Plaques and Tangles That Cause Alzheimer’s
Meryl Comer: The mainstream research has been focused on beta amyloid. Tau has always been there, but now there is a big controversy about where the progression comes, where does it really lie? Take that debate on Dr. Gandy.
Dr. Gandy: Well certainly people with Alzheimer’s disease have two sorts of lesions in their brains, two sorts of clumps of protein. Some of these clumps are in between nerve cells, and others are inside nerve cells. The ones that are in between the nerve cells are called amyloid plaques. The clumps that are inside the nerve cells, which are twisted, are called tangles or neurofibrillary tangles. Now for many years we didn’t sort of know what the sequence of events was, but it is very clear now that all the genes that cause Alzheimer’s disease point to the buildup of amyloid. So it appears that Alzheimer’s disease amyloid comes first and tangles come next. They may be extremely important in understanding why the nerve cell dies. Now the disease that Dr. Troncoso mentioned, frontal temporal dementia, has also helped us to understand the relationship between plaques and tangles because in that disease the mutations that cause the genetic forms are in the protein called tau that builds up and causes tangles. People with frontal temporal dementia get tangles, but they never get plaques, so in Alzheimer’s disease plagues can cause tangles, but in frontal temporal dementia tangles don’t cause plaques.
Meryl Comer: Well why is it so hard these days to get a grant from NIA around beta amyloid when you can get it for tau?
Dr. Gandy: Well so there is a specific reason for that that’s really evolved a lot in the last year. There is a study that was reported this spring that showed using an antibody, a chemical aimed at the amyloid substance... that if people with mild Alzheimer’s disease received antibody infusions, anti-amyloid infusions, for a year and a half that the amyloid buildup in their brains would go down by about 25%. They didn’t change at all clinically. They didn’t get any better in terms of their cognitive function. Why is that? Because we didn’t start early enough, because we didn’t treat long enough or because it’s actually another form of amyloid, not the plaques, but these floating clumps called oligomers?
Meryl Comer: You wanted to make a point, yes, doctor.
Dr. Troncoso: Yeah well, I think that there is a lot of debate between the amyloid and tau deposition, but I think one should not get stopped at that point of that argument because it’s perfectly possible that one of these abnormalities, let’s say amyloid may trigger the rest and there is more than amyloid and tau. We haven’t spoken, but there is a very significant inflammatory, inflammation in the brain that once you have perhaps amyloid and tau trigger that event it becomes self-sufficient. It actually may even promote more amyloid or more tau deposition, so I think that tau it may be as important as amyloid, but it may be later on in this progression of the disease. And if you could actually target each of these elements it probably would be beneficial. So I don’t see really a tremendous dichotomy, antagonism between looking at amyloid and tau. I think that both are perfectly legitimate targets of research and one more perhaps disgression in terms of the dementia that is being seen in patients who have head trauma. Most of that, the lesions that they have is of the tau type, so I think both of these targets amyloid and tau should be addressed. There is no reason to eliminate one of them.
Dr. Gandy: There is the one experiment to mention that might also explain why the shift sort of toward tau. A lot of what we’ve learned about Alzheimer’s disease is from mouse models. Mice normally never ever get Alzheimer’s disease because their amyloid is different enough that it doesn’t clump and build up. If we then put into a mouse the gene for amyloid and with a mutation that would cause it to build up and the gene for tau so that it will get tangles, then as that animal ages it will get buildup of plaques and tangles just like, similar to humans with Alzheimer’s disease. They will then lose their ability to find their way around their cage or to find their way around a swim maze. If you then treat them with a drug or substance that will decrease the levels of tau, will lower the tau down, the cognitive function comes back, so it’s possible to sort of render the amyloid inert if you can turn down the tau at least in the mouse model.