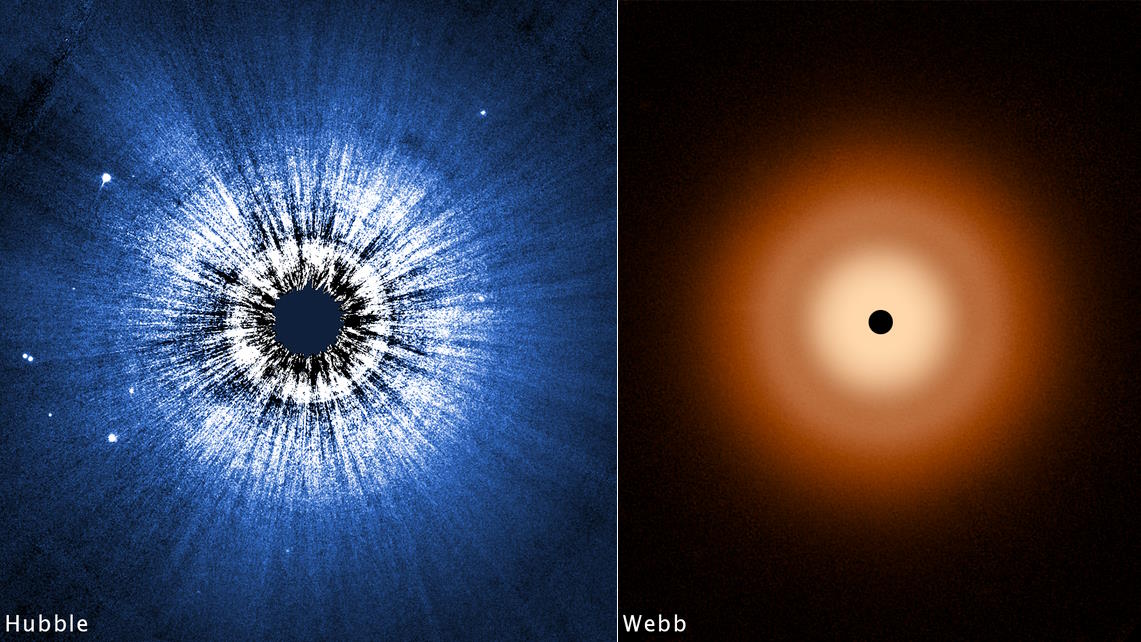
The Cancer Genome Atlas project, already several years underway, is transforming the way scientists think about and treat cancer.
Siddhartha Mukherjee: Tell us a little bit about what the Cancer Genome tells us about the genetics of cancer and what we have learned from the Human Genome Project followed by the Cancer Genome Project. Do you want to start and then we’ll go around?
Dr. Lewis Cantley: Sure, well, as I said earlier the first thing the Cancer Genome is beginning to tell us is that there are many, many more subdivisions of cancers than we previously could predict based on pathology alone, and I think that is going to be extremely informative. The goal now with target therapies—first of all, it’s telling us what genes are likely driving the cancer, so if you find that in lung cancer 30% of the people get a particular mutation in a gene called RAS, 90% of pancreatic cancers have exactly that same gene, and you see that those are exactly the subgroups that don’t respond to any of our existing therapies. Then you realize that we need to have drugs that target RAS in some way. All the approaches we’ve made thus far have failed for that particular target, so people are trying to figure out ways to do that; it turns out to be a difficult gene to target. So the first thing that is going to come out and has already come out is the identification of what we call drugable oncogenes, things that are mutated in the cancer EGF receptor in lung cancer for example. So those opportunities hopefully we already know a lot about those and drugs are being developed and we’re hoping to get a lot of success out of that. In some ways I think on the other hand it has been somewhat disappointing in that most of the things we’re finding from the cancer genome sequencing are things that we already knew. In some ways retroviruses that cause cancers in mice and chickens, things that Harold actually worked on as a post doc and helped develop the field; they’ve essentially done the experiments in other animals and identified the oncogenes for us, so we’re finding relatively few oncogenes that we didn’t already know about from these mutational events, but I think it’s still going to be extremely valuable.
Dr. Siddhartha Mukherjee: Do you share the disappointment about the Cancer Genome Project Harold?
Dr. Harold Varmus: No, but I would point out a few things building on what Lou just said. First we knew about the RAS genes of course well before the Cancer Genome Projects, but we knew about RAS genes and the mutations that arise in them very frequently in pancreatic, lung and colon cancer even before there was a Human Genome Project because we knew about that in the early 80s, so I think it’s useful to remind people of that because here is a tremendously attractive target for developing drugs, even for thinking about ways to diagnose cancers early and we really have had a hard time capitalizing that over the last 30 years and that is a good reminder of how difficult this all is.
The second point I’d make is that, yes, I agree that we have of course rediscovered in the Cancer Genome Atlas Project and other efforts of genomics of cancers that the oncogenes we knew about because they’re relatives of the retroviral cancer genes, sure, they’re involved quite frequently as our well know so called tumor suppressor genes. But we’re getting a much better picture of what the real complexity of cancer might be. Secondly, there are new methods that extend our ability to analyze a genome well beyond just looking for small mutations. There are some very important rearrangements. We were talking earlier about prostate cancer and one of the things that I think is a pretty good indicator of the danger of a prostate cancer can be seen by finding a rejoining of chromosomes that generates a new gene that seems to be a driver of prostate cancers and that's the kind of thing that would have been very difficult to discover without this concerted effort that is going on.
Third, there are actually changes in the genome that are not mutational. They affect the way DNA is modified by chemical processes and the way in which DNA is expressed because of changes in the proteins that coat DNA and allow genes to be expressed. Very recently there have been a surprising set of mutations that govern what we call the methylations, a kind of chemical addition to DNA that governs gene expression that is regulated by a series of enzymes and coated by genes we would not historically have thought of as oncogenes and it’s clear from some recent work about an adult leukemia called acute myeloid leukemia that these genes are playing a very important role in that disease and represent another kind of target for therapy and a way to think about diagnosis, so I think there are a lot of rather surprising things that are coming out of this and the picture, the full picture of genomic change is really very dramatic and quite wonderful.
Dr. Lewis Cantley: So if I could just add one additional thing. I don’t want to leave the impression I'm disappointed or we shouldn’t have done that, and of course the mutations that Harold was talking about I think are very exciting. It really has opened up the field, but it’s still a relatively minor subset of cancers that are involved in gliomas and AMLs.
Dr. Harold Varmus: Well I think we don’t know yet.
Dr. Lewis Cantley: Well there is- Yeah, anyway, that is definitely helping us out, but I think the other thing that we’re going to get from this is biomarkers that will allow us to do much smarter clinical trials, so that we do a clinical trial and 15% of the patients respond. If you don’t have thousands and thousands of patients it’s hard to prove that that 15% is actually relevant and the drug may fail to be approved even spite of a huge investment in the trial and many, many years and there is examples of that that we know of where it was very clear to the clinicians the drug was working, but it still didn’t get approved, so if we can design trials where we tease out early on who is going to respond and who isn’t then that 15% becomes the 100% because you only do the trial on them. They’re defined by their mutational status, the so called biomarkers that- these are biomarker-driven trials, so I think that is going to be a tremendous advantage coming out of this.
Dr. Harold Varmus: If I can make one comment about the clinical trials because this is of great interest to the public. One reason we were so successful with pediatric cancers, developing chemotherapies that cure a large fraction of pediatric cancers is because virtually every kid came to a cancer center, was entered in a clinical trial and over the course of many years a lot of disappointments, heartache, and a lot of loss of life because these cancers are very, very difficult to treat, we finally emerged with a set of principles and operating procedures for treating these kids effectively. We’ve been much less successful with adult therapy and one of the reasons I think is because the heterogeneity of the tumors as Lou is describing. The Cancer Institute is currently reorganizing its clinical trial system in a way that is designed to take advantage of the point that Lou is making. For example, in treatment of lung cancer we know that a small fraction are susceptible to inhibitors of mutant kinases and one of the drugs erlotinib just barely squeaked through its first clinical trial because they were enough patients with that mutation in their tumors. Another very similar drug didn’t squeak through because it had too many people who didn’t have that mutation and now that the Cancer Genome analysis is giving us the tools to so called stratify these patients I think all of us believe there can be much smarter trials set up, faster trials, trials that give us much more effective information about how to treat.