microbes
In his new book, the popular science writer tells the story of how scientists discovered the “gaseous ocean” we all swim in — and the trillions of invisible life forms we share it with.
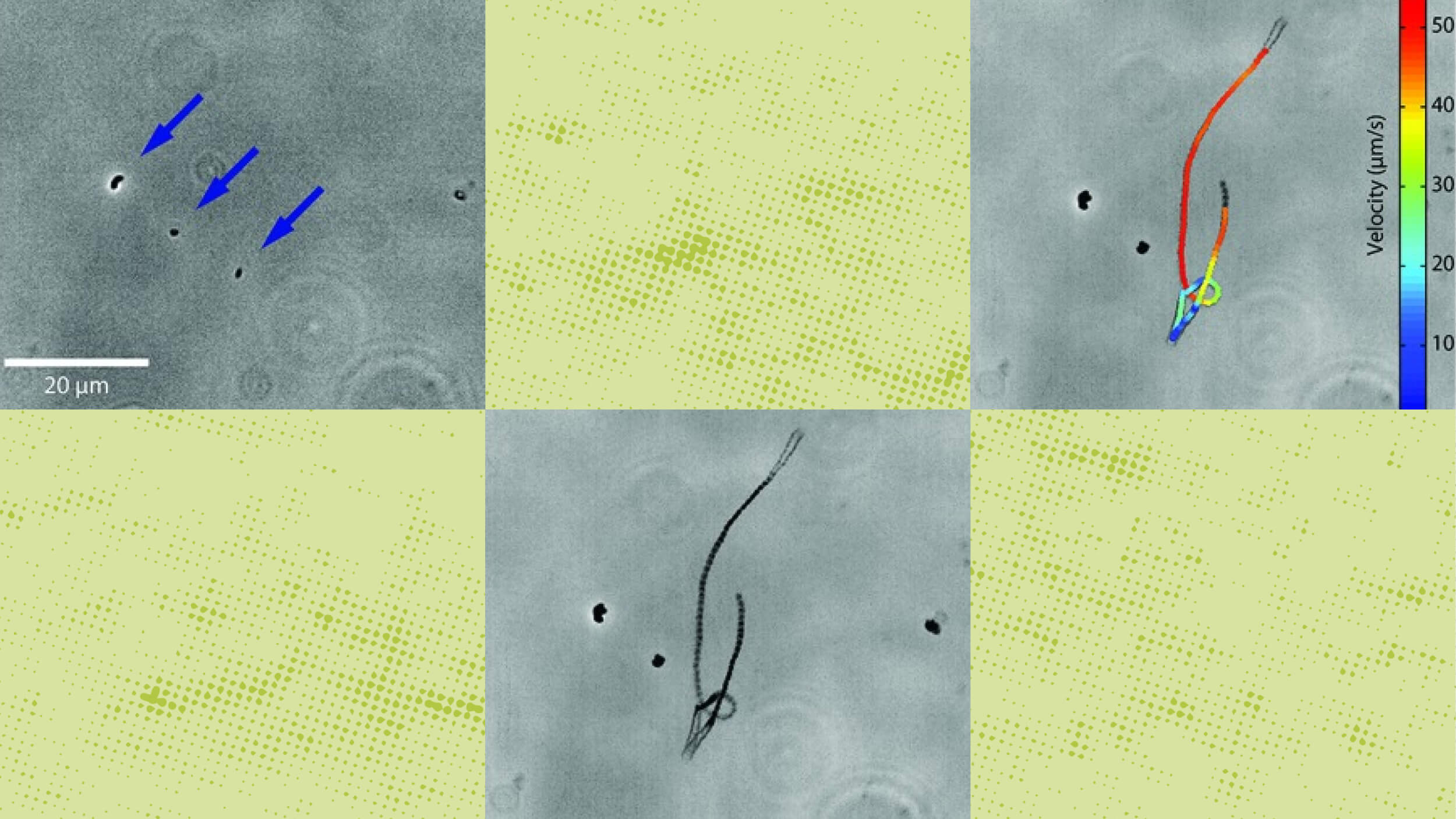
Motility was suggested as a promising “biosignature” as early as the 1960s, but the technology was insufficient — until now.
Caitlin Rivers wants to tell the story of epidemiology and the public health heroes who keep the world safe and healthy.
Could studying the Oriental hornet lead to a treatment for people with alcohol use disorder?
We need a “theory that explains the evolution of evolution,” argues theoretical physicist Sara Imari Walker.
Differences in certain avian and mammalian proteins explain why avian influenza doesn’t (typically) infect humans.
Scientists might be looking for Martian life in the wrong place.
The recent discovery of a large cave on the Moon highlights the importance of caves not just for future space explorers but astrobiology as well.
A long view of biological survival might point us to new possibilities for finding life elsewhere in the Universe.
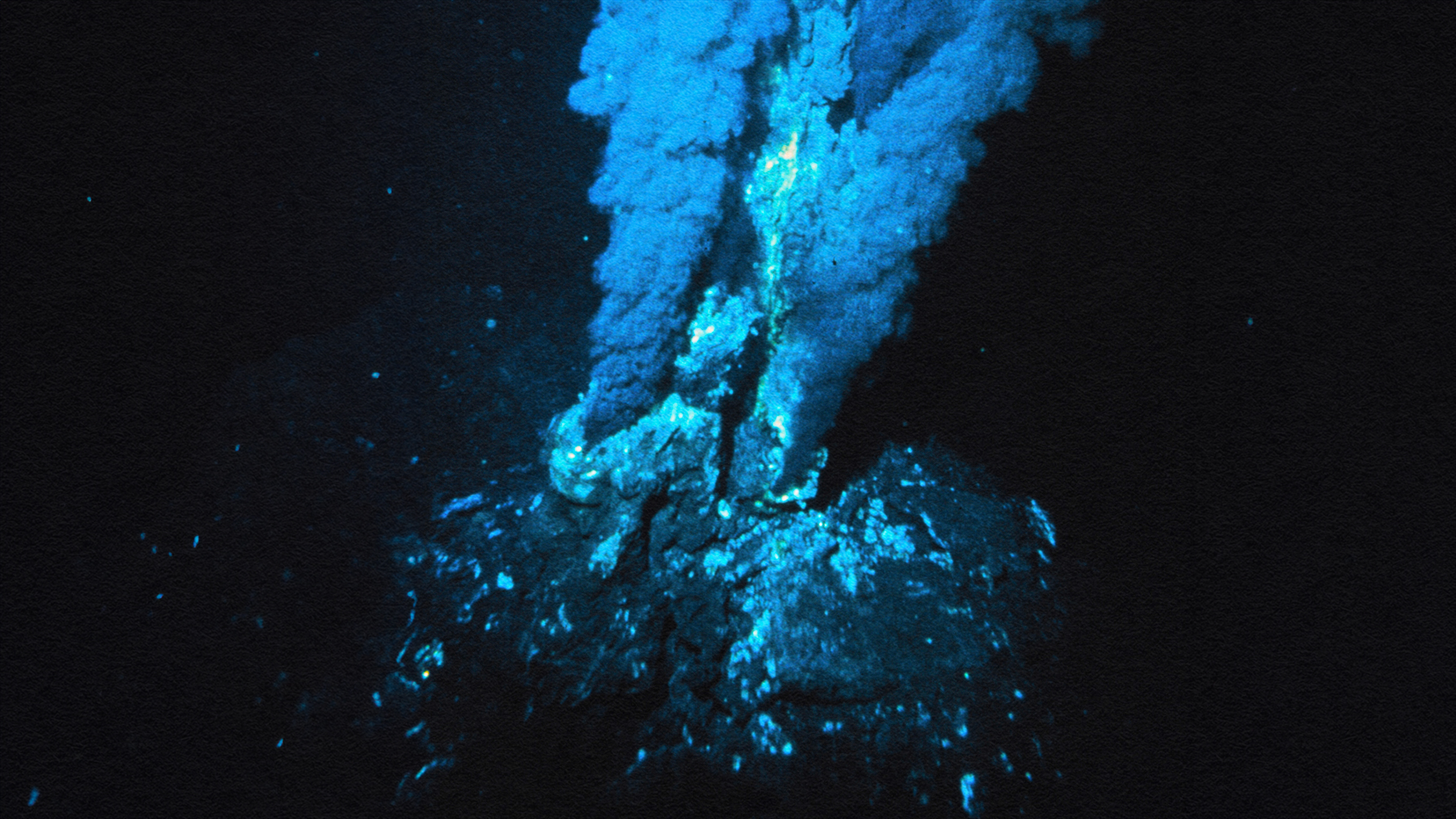
This research team is working out how to detect extraterrestrial cells in the liquid water ocean hidden beneath Enceladus’s icy crust.
SARS-CoV-2 first emerged in humans in 2019. Despite much noise generated by lab leak proponents, the evidence indicates a natural origin.
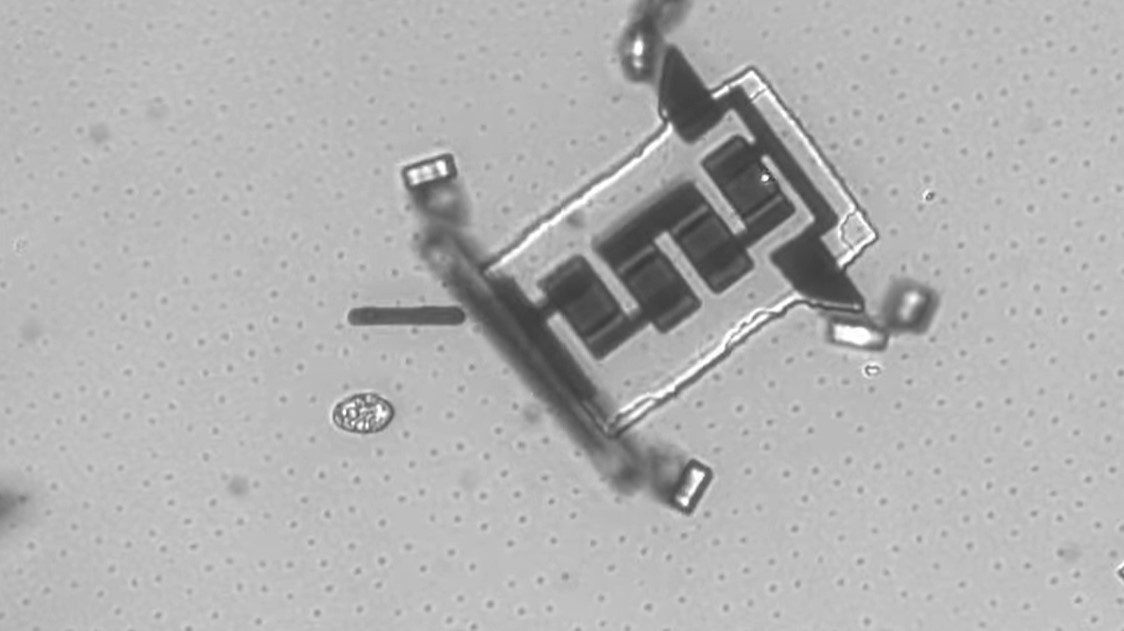
What would it take to create a truly intelligent microbot, one that can operate independently?
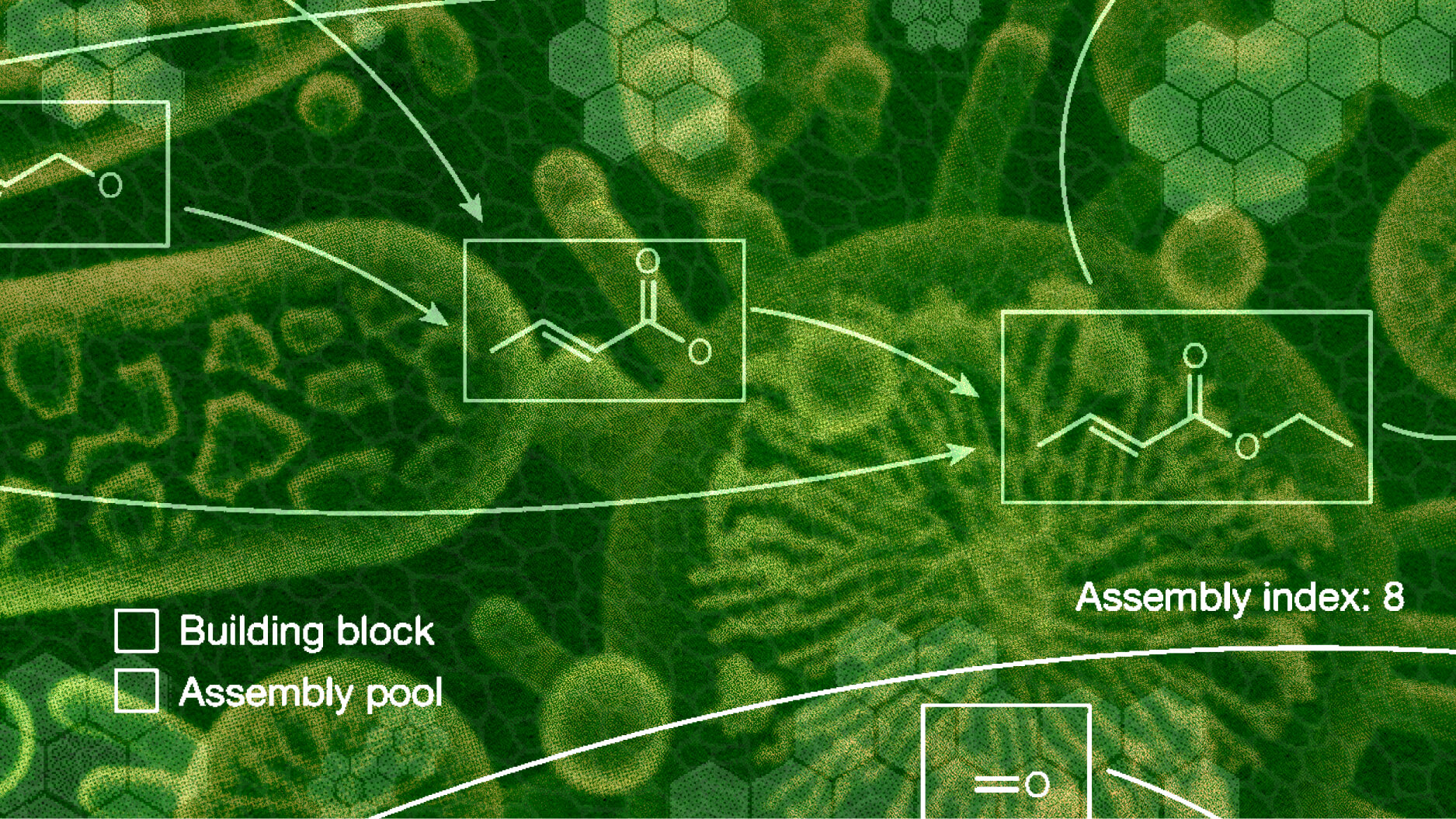
In a recent paper, biologists outlined a three-part hypothesis for how all life as we know it began.
We don’t yet know if these strange “obelisks” are helpful or harmful.
Although early Earth was a molten hellscape, once it cooled, life arose almost immediately. That original chain of life remains unbroken.
New DNA analyses raise questions over the theory that Christopher Columbus and his men brought syphilis to Europe.
Research suggests that to maintain a healthy brain, we should tend our gut microbiome.
Life became a possibility in the Universe as soon as the raw ingredients were present. But living, inhabited worlds required a bit more.
Growing evidence suggests a link between the debilitating neurological illness and the microbes that live in our intestines. The vagus nerve may be a pathway.
Earth wasn’t created until more than 9 billion years after the Big Bang. In some lucky places, life could have arisen almost right away.
ÄIO’s fermentation process creates healthy, sustainable oils and fats by upcycling low-value industry organics.
Artificial intelligence can forecast the behavior of viruses and quickly make vaccines to thwart them.
Antioxidant vitamins don’t stress us like plants do—and don’t have their beneficial effect.
EBT-101 is not the only candidate for an HIV cure. Stem cell transplants, medications, and other CRISPR therapies are being researched.
AI was key to making Moderna’s COVID mRNA vaccine. Its role in mRNA therapeutics will rapidly grow in the coming years.
With such a vast Universe and raw ingredients that seem to be everywhere, could it really be possible that humanity is truly alone?
The best answer we have is, “Life is matter with intentionality.”
CRISPR, stem cells, and even cancer drugs are helping shape an AIDS-free future.
It’s early days, but if the efforts can be efficiently scaled-up, such biological recycling could put a dent in the plastic waste problem.
Over a third are worried that vaccines can cause “canine autism.”