Sodium and water react, and quantum physics explains why

- Take a chunk of sodium metal and get it wet, or drop it in water, and not only does it fizz and give off heat, but it can even produce flames and explosions.
- It’s easy to say, “it’s just a chemical reaction,” but this reaction, at an atomic and molecular level, is governed by the rules of quantum physics.
- It has everything to do with ionization energy, and how easy it is for sodium to lose an electron compared to water, hydrogen, or oxygen. The result is nature’s spectacular show.
It’s a little bit paradoxical: when we encounter something unexpected for the first time, we wonder why it happens that way. But if we encounter a phenomenon often enough, even if it ought to surprise us and cry out for an explanation, we simply accept that this is how the world works.
Drop a salt crystal — simple sodium chloride — into water, and it just dissolves.
Drop some chlorine into water, and you can disinfect it: killing bacteria, viruses, and other disease-causing microorganisms present within.
But if you drop sodium into water? The reaction that ensues is legendary in its violence.
As soon as you get that chunk of metal wet, the reaction fizzes and heats up, the sodium bounces around on the surface of the water, and even flames are produced. Sure, it’s just chemistry. But at a fundamental level, there’s something more at play: there are quantum interactions that take place between the sodium metal and the water molecules (and its dissociated ions) that can immediately ensue. Although it’s tempting to say, “it’s just chemistry,” the physical reason behind this reaction is fascinating and informative, reminding us that we should remain curious about even the mundane phenomena we’ve grown accustomed to in the Universe.
Although there are many ways to think about atoms, the chemical reactions that take place between sodium and water makes the most sense when you think about atoms as “noble gases” with extra protons in their nucleus and extra electrons in their valence shells. Sodium, for instance, is very similar to the noble gas neon, the tenth element on the periodic table, which has ten protons in its nucleus and has both its first (1s, with two electrons) and second (2s, with two electrons, and 2p, with six electrons) orbitals filled with ten electrons total.
Noble gases are famed for not reacting with anything, and the reason is that all of its occupied atomic orbitals are completely full of electrons. That ultra-stable configuration gets ruined when you go up by one element on the periodic table, and this happens for all the elements that fit this pattern, including sodium. With one extra proton in its nucleus, those filled electron orbitals are held onto more tightly, but then that last extra, valence electron is only held onto very loosely. Helium is ultra-stable, but lithium is highly reactive. Neon is stable, but sodium is reactive. And argon, krypton, and xenon are stable, but potassium, rubidium, and cesium are reactive.
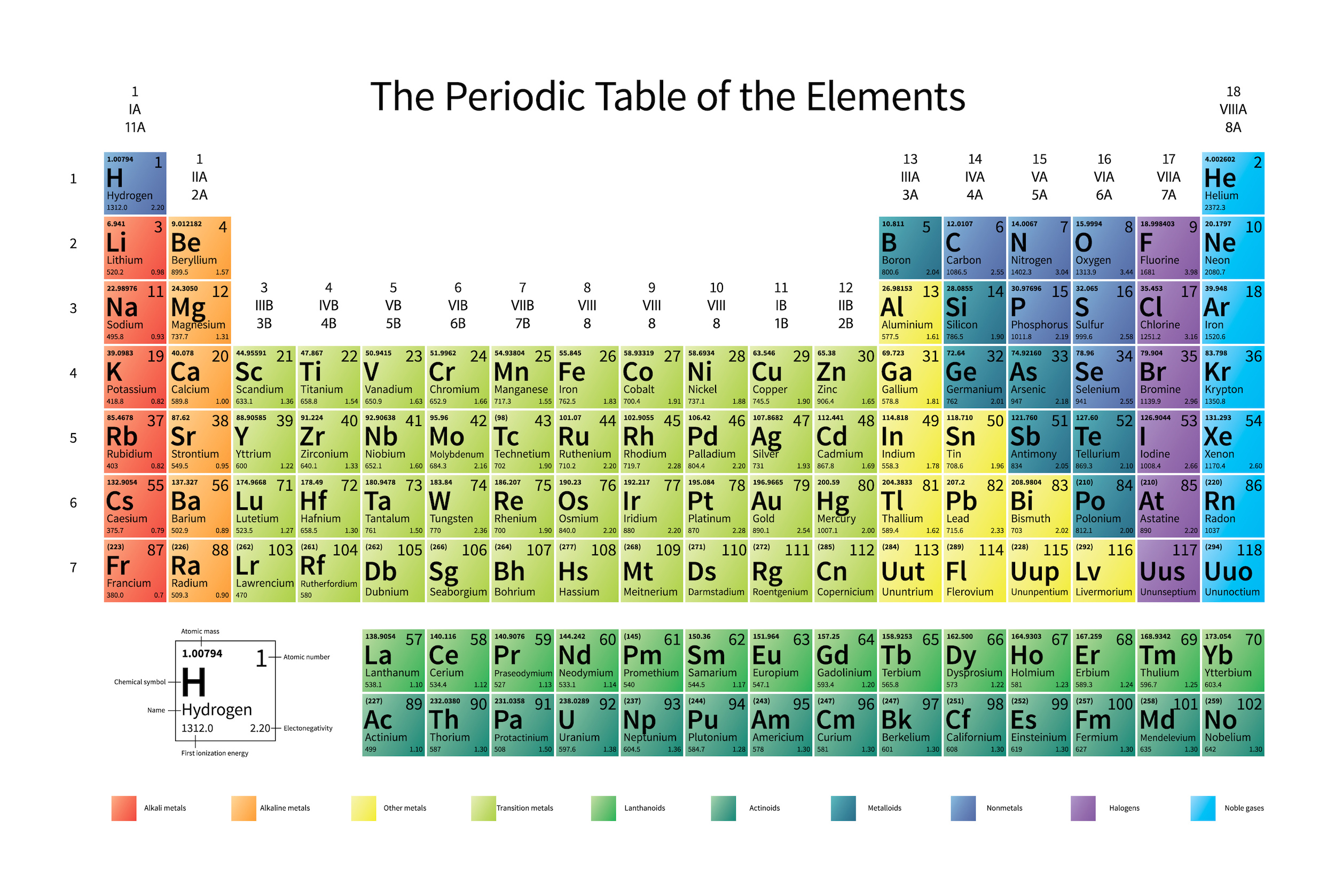
The reason for the extreme reactivity? It’s the extra electron.
When we learn about atoms, we learn to think about the nucleus as a hard, small, positively charged core at the center, and the electrons as negatively charged points that orbit it. But in quantum physics, that’s not really the whole story. Electrons can behave like points, particularly if you fire another high-energy particle or photon at them, but when left to their own devices, they spread out and behave like waves. Those waves can configure themselves in particular fashions:
- spherically (for the s-orbitals, which take 2 electrons each),
- perpendicularly (for the p-orbitals, which take 6 electrons each),
- and so on up through the d-orbitals (taking 10 electrons),
- the f-orbitals (taking 14),
and beyond, following the pattern first identified by Mendeleev.

The reasons these shells fill up is due to the Pauli Exclusion Principle, which prevents any two identical fermions (like electrons) from occupying the same quantum state. Because electrons possess a fundamental quantum mechanical property called spin — a measure of the electron’s intrinsic angular momentum — and an electron’s spin can be either +½ or -½, each unique quantum state can have two electrons within it: one that’s spin +½ and one that’s spin -½.
In an atom, once an electron shell or orbital fills up completely, the only place to put an additional electron is in the next orbital up. An atom like chlorine or fluorine will readily accept an additional electron, since it only requires one more to fill its electron shell; conversely, an atom like sodium or potassium will readily give up its last electron, since it has one extra electron over what will fill a shell. This is why sodium-chloride (NaCl) is such a good salt: the sodium gives up an electron to the chlorine, and both atoms subsequently exist in a more energetically favorable configuration.
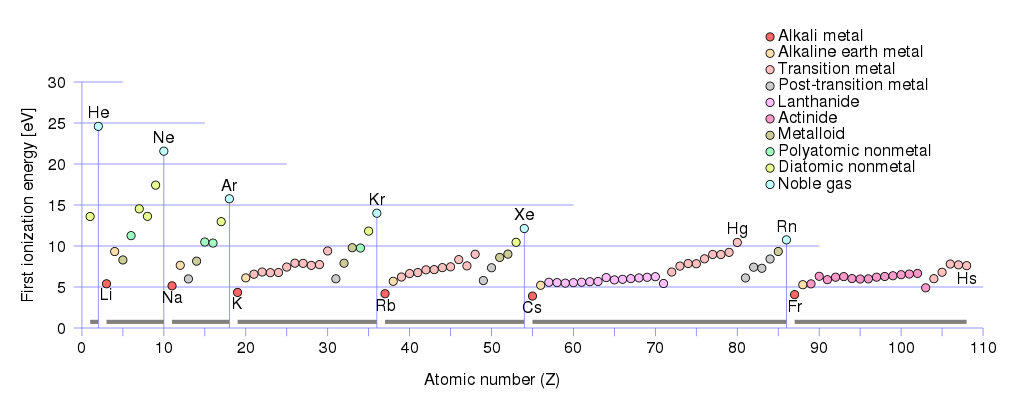
In fact, the amount of energy required for a neutral atom to give up its outermost electron, known as its first ionization energy, is especially low for all of those metals with one valence electron. If you look at the numbers, it’s much easier to strip a single electron off of lithium, sodium, potassium, rubidium, cesium, etc., than any other element.
Moreover, as we look to progressively heavier elements — ones with larger numbers of protons in the nucleus overall, and hence, with greater numbers of completely filled orbitals — we find that the overall ionization energy decreases dramatically.
This property plays an important role, believe it or not, in the formation of new stars. When the first stars formed, the Universe consisted only of difficult-to-ionize hydrogen and helium, so unless you heated those elements up to hot enough temperatures to ionize them, they only cooled gas clouds down very slowly. This results in large-mass clouds that form higher-mass stars; later on in the Universe, when there are greater abundances of heavier elements, cooling happens far more easily owing to the abundance of more easily-ionized molecules. Later stars can form from lower-mass clouds, and result in lower mass stars than their earlier, more pristine counterparts.
So what is it that happens, then, when you bring a sodium atom — an easily-ionized sodium atom that only holds onto its outermost electron relatively weakly — into the presence of water?
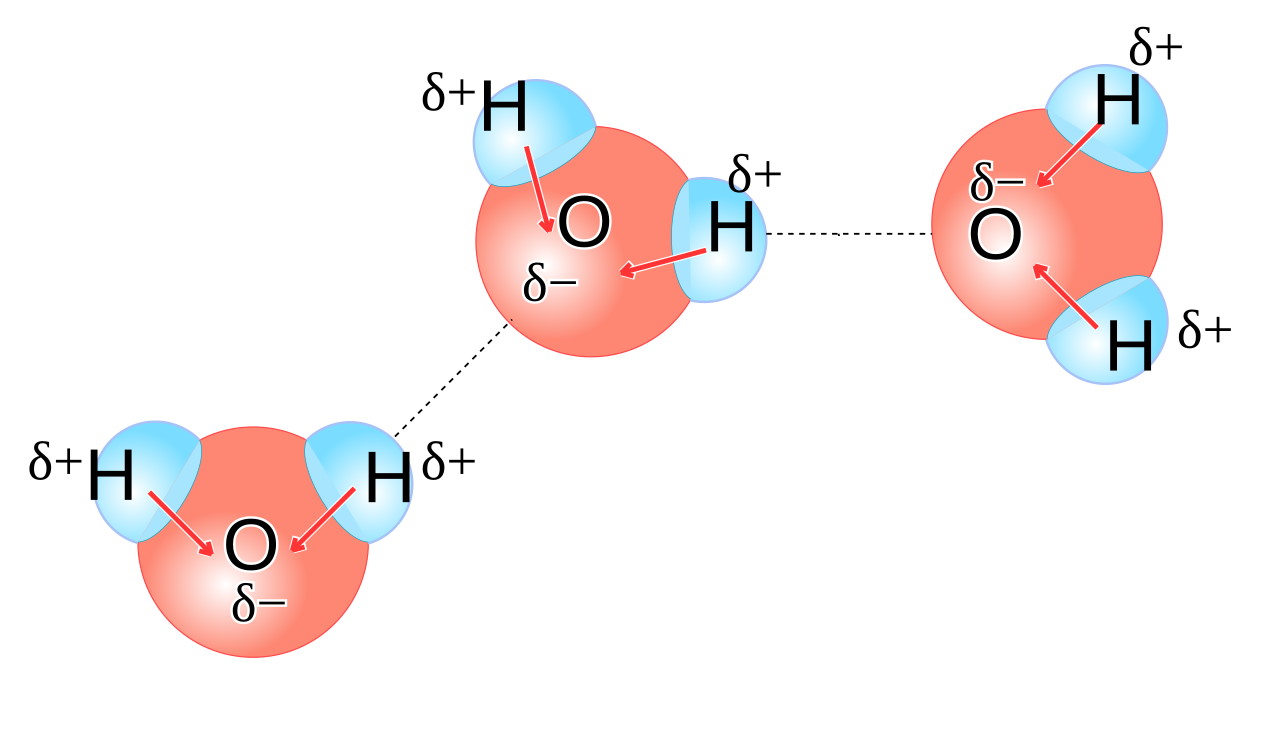
You might be tempted to think of water as its own very stable molecule: H2O, with two hydrogens bonded to one oxygen. But water is a highly polar molecule, meaning that one side of an H2O molecule (the side facing away from the two hydrogens) has a preferentially negative charge, while the opposite side has a preferentially positive charge.
This means that each water molecule has a “negatively charged” end, where the oxygen is located, and a “positively charged” end opposite to that. When you have liquid water, these molecules line up relative to one another in such a way that the electric potential energy is minimized, and the negative end of one water molecule is more likely to wind up attracting the positive end of other water molecules. It’s a significant enough effect that it causes some water molecules — about one in a few million or so — to dissociate into two ions: a single proton (H+) and a hydroxyl ion (OH–).
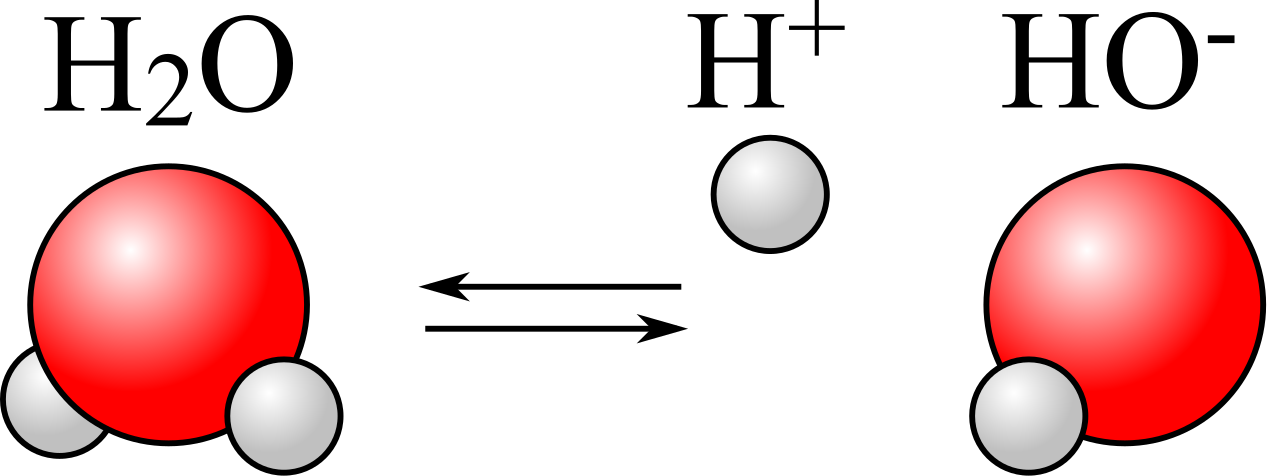
This has a lot of consequences for things like acids and bases, dissolving salts, activating chemical reactions, etc. But what happens, then, when you have even pure water, made of not only H2O but also containing a small (a little less than 0.0001%) percentage of both protons (H+) and hydroxyl ions (OH–), and then you add neutral sodium to it?
Sodium, this neutral atom with a loosely-held outermost electron, is now in the presence of water: neutral but polar water, free protons, and hydroxyl ions. What we have to ask, then, is which configuration is the most energetically favorable?
- Will sodium give up its outermost electron to neutral water?
- Will sodium give up its outermost electron to the hydroxyl ion?
- Will sodium give up its outermost electron to the free proton?
- Or will sodium keep its outermost electron, or even gain an electron from one of the other particles?
The answer is a no-brainer; in pretty much every case, the electron will jump from the sodium atom to the first single proton it finds.
In fact, it’s not even much of a contest. At the scale of individual atoms and ions, we typically think about the answers to questions like “How much energy does it take to ionize an electron?” or “How much energy gets liberated when an electron finds an ion and makes a ground-state, neutral atom?” in energies on the scale of electron-volts (eV). It takes a little more than 5 eV of energy to ionize a neutral sodium atom, and when a sodium ion gains an electron, it liberates that same ~5 eV of energy. But it takes over 13 eV of energy to ionize neutral hydrogen, and that same 13.6 eV of energy is liberated when a hydrogen ion becomes neutral again.
That’s why the reaction happens so quickly when sodium metal is dropped into water, and why it gives off so much energy. In fact, as the electrons given up by sodium start to combine with hydrogen ions, the neutral water further dissociates into free protons and hydroxyl ions, creating even more “fuel” for the sodium to react with.
But that doesn’t complete the story, either. Now that we’ve made neutral hydrogen atoms, they don’t just form a block of individual atoms that you can bind together like sodium metal does. Instead, at room temperatures and pressures, hydrogen is a gas, and goes toward an even more energetically favorable state: forming the neutral hydrogen molecule, H2. So now, you’ve got lots of free energy (which goes into the heat of the surrounding molecules), neutral hydrogen gas, and it rises up out of the aqueous solution and into the atmosphere, which is mostly neutral nitrogen (N2) and oxygen (O2) gases.
Do you recognize that recipe?
You have lots of free energy, in the form of heat. You have molecular hydrogen gas, and you have molecular oxygen gas.
That’s a surefire recipe for combustion! Hydrogen/oxygen combustion is a key component of rocket fuel, of hydrogen fuel cell vehicles, and of the famous Hindenberg disaster. (Which is why we fill blimps nowadays with inert helium instead of combustible hydrogen!)
When oxygen and hydrogen react in the presence of energy, this fiery combustion reaction produces water vapor, but also gives off even more energy. This explains why, when you drop a large enough chunk of sodium (or any group 1 element from the periodic table) into water, you get that tremendous, explosive release of energy. It’s all driven by the transfer of electrons, which occurs due to the quantum rules governing the Universe, and the electromagnetic properties of the charged particles that make up these atoms and ions.

So to recap, when you drop a chunk of sodium into water, here’s what happens.
- First, the sodium immediately gives up its outermost electron to the aqueous solution that is water.
- Next, that electron is absorbed by a hydrogen ion, forming a neutral hydrogen atom.
- That initial reaction liberates a large amount of free energy, causing the surrounding molecules to heat up.
- Immediately afterward, the neutral hydrogen atoms bind together to rapidly form copious amounts of molecular hydrogen gas.
- Hydrogen gas is much lighter and less dense than the other atmospheric gases, and so it rises out of the aqueous solution.
- And finally, if there’s enough energy, the atmosphere’s oxygen reacts with the hydrogen gas, creating a combustion reaction.

This will continue until the sodium metal is all gone, or rather, until the sodium is fully dissolved, in ionized form, in the remaining water. It’s not just chemistry at work; it’s the underlying science of quantum physics that makes it all possible.
The very rules that govern the behavior of every one of these chemical reactions arise from even more fundamental laws:
- those of quantum physics, including the Pauli exclusion rule which governs the behavior of electrons in atoms,
- and those of classical electromagnetism, which governs how charged particles interact.
It’s not often that you can derive the full behavior of a complex system from the simple, fundamental rules that underpin their constituent components, but in the case of sodium reacting with water, we can do precisely that. Without these physical laws and forces, we’d have no chemistry at all! Yet thanks to them, any time you drop sodium into water, you know exactly what to expect. And if you still don’t know what to expect when you drop sodium into water, the answer is to wear protective equipment, don’t handle the sodium with your own water-containing hands, and stand back at a safe distance while the reaction occurs!