The act of ice skating is a miracle of physics

- From graceful figure skaters to brutish ice hockey players, the act of wearing ice skates can transform a frozen pond from a nightmare to navigate into a dream.
- Although ice itself is normally a slick, low-friction surface that's very hard to steady yourself on and move across in a controlled fashion, the act of ice skates makes it not only possible, but easy.
- This is due to a combination of properties that are specific to water and its solid form, ice, that are only rarely found in nature. The ability to ice skate truly is a miracle of physics.
Imagine there’s a large, flat sheet of ice out in front of you, and someone unceremoniously shoves you across it at a high speed. What are you to do? If you’re wearing conventional shoes, without crampons or blades attached to them, you’re going to have a difficult time. Ice is a very low-friction surface, and there’s very little you’re going to be able to do to change your momentum without slipping and perhaps falling down. You’re bound to simply slide along until either you run into an obstacle or slowly come to rest, likely a long way from where you began.
But if you put thin blades on the bottoms of your shoes — e.g., wear ice skates — you’ll discover that the situation is very much different in this case. As long as you can remain on your feet, with only your blades touching the ice, you’ll find that you can control your motion relatively easily, simply by applying forces through your feet (and the blades) to the ice down below. You can speed up, slow down, or change direction at will, and only if you fall or lose control of your skates (and body) will you wind up in a similar situation to the no ice skate case. It might seem miraculous, but there’s physics behind what you’re experiencing at each and every step. Here’s how it all works.
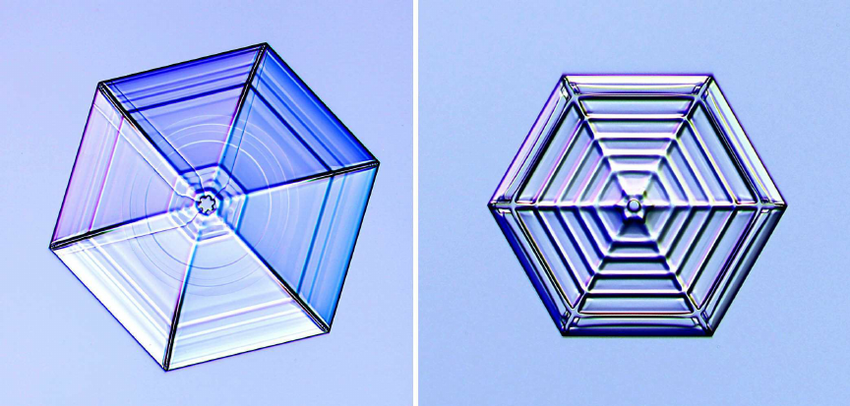
Here on the surface of Earth, at normal atmospheric pressure and wherever you have sub-freezing temperatures, almost all of the ice you’ll encounter comes in a very specific configuration: normal hexagonal crystalline ice, sometimes known as Ice Ih. Ice, just like water, is made up of primarily a very simple molecule (H2O) with two hydrogen atoms anchored by a single oxygen atom, and with a very specific bond angle between them. Whereas in liquid water, the bond angle is 104.5° between the connecting lines of each O-H bond, in normal hexagonal crystalline ice, the bond is lengthened into the shape of a more perfect tetrahedron: 109.5°.
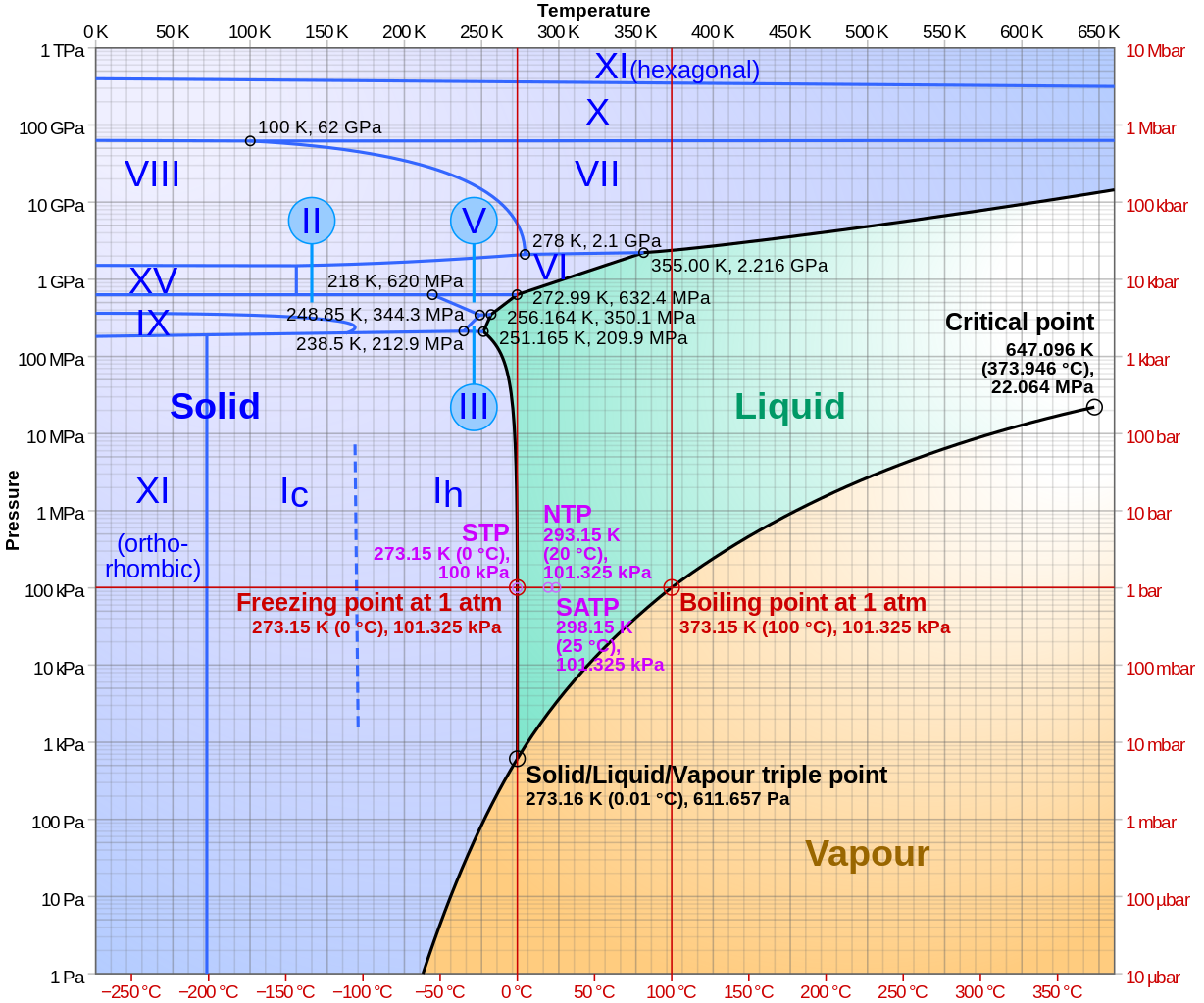
Under different temperature and pressure conditions found on Earth and elsewhere in the Universe, different possibilities arise for how those various molecules bind together, creating a massive variety of possible configurations. At present, there are a whopping 20 known phases of ice, including:
- Ice Ih, which is normal hexagonal crystalline ice and the most common form of ice found on or near Earth’s surface,
- Ice Ic, which is a cubic crystalline variant of ice whose oxygen atoms are arranged in a diamond structure, that often appear at the lower temperatures found in the upper atmosphere,
- And amorphous ice, which has no crystalline structure and is sometimes formed at ambient atmospheric pressure,
in addition to the higher-pressure/temperature phases of ice: Ice 2 through Ice 18.
However, it’s the most common form of ice, plain old Ice Ih, that’s relevant for the problem of ice skating. Normally, under this configuration, the water molecules within ice are arranged in a hexagonal crystal lattice, and adding new water molecules to these icy structures will simply result in the growth of the main crystal in the same ongoing pattern. Adding more molecules won’t change the structure of your ice, it will simply cause those new molecules to bind together to the available spaces open in the underlying lattice, keeping the same “type” of ice but just making more and more of it.
If you want to change the form of ice that you have, you can always either heat it or cool it, or apply a varying amount of pressure to it. Those changes in temperatures and/or pressures can often coax ice into a new configuration, as it’s sometimes more energetically favorable for those molecules (and the atoms within them) to arrange themselves into a different form of ice.
However, the most common fate of hexagonal crystalline ice — the type of ice found on Earth’s surface — is that either heating it or compressing it will simply cause it to melt.
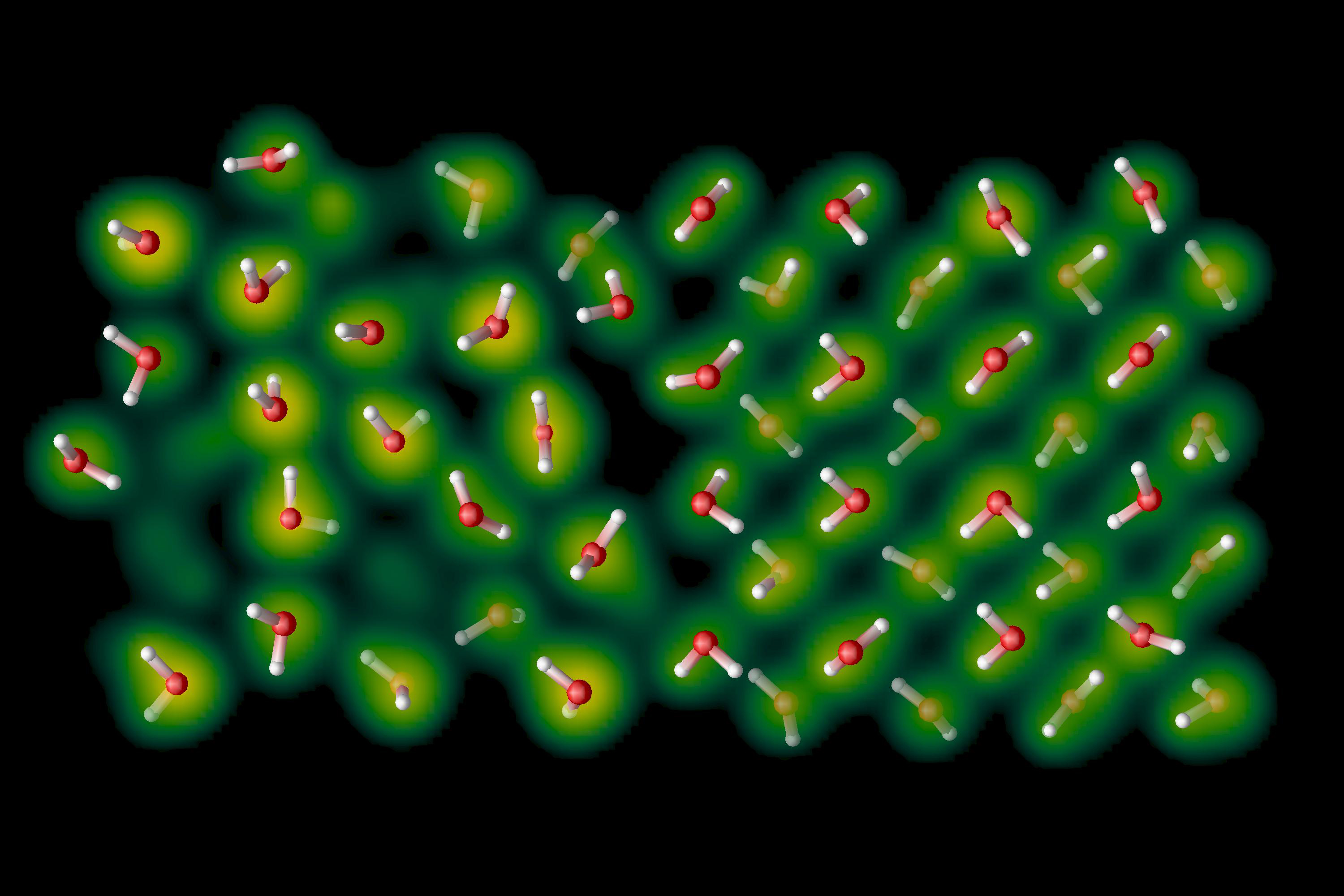
Melting ice by raising its temperature (or applying the addition of heat) is probably the circumstance that most of us are most familiar with. If you take an ice cube out of the freezer, the ambient heat in the surrounding air in your house is usually enough to trigger the onset of melting. If you drop ice into a reservoir of liquid, such as in a glass of water, juice, or alcohol, heat will be transferred into the ice cube and will begin the melting process. When that ice melts, the now-liquid water molecules are no longer bound to be in the crystalline structure that made up ice previously, but can instead rotate, vibrate, and slide their way into any imperfection in the ice through natural processes.
This is something you might have noticed if you’ve ever played in the snow and ice before. When the ice is very hard and no heat is applied, it often possesses sharp edges and corners, and might feel more like a salt or quartz crystal in your hands. However, because your hands (for all of your warm-blooded mammals out there) are generally at a temperature that’s above the freezing point of liquid water, continuing to keep that ice or snow in your hands will “smooth out” the ice, as the most jagged edges are what you melt first, leaving the smoother portions of the ice intact.

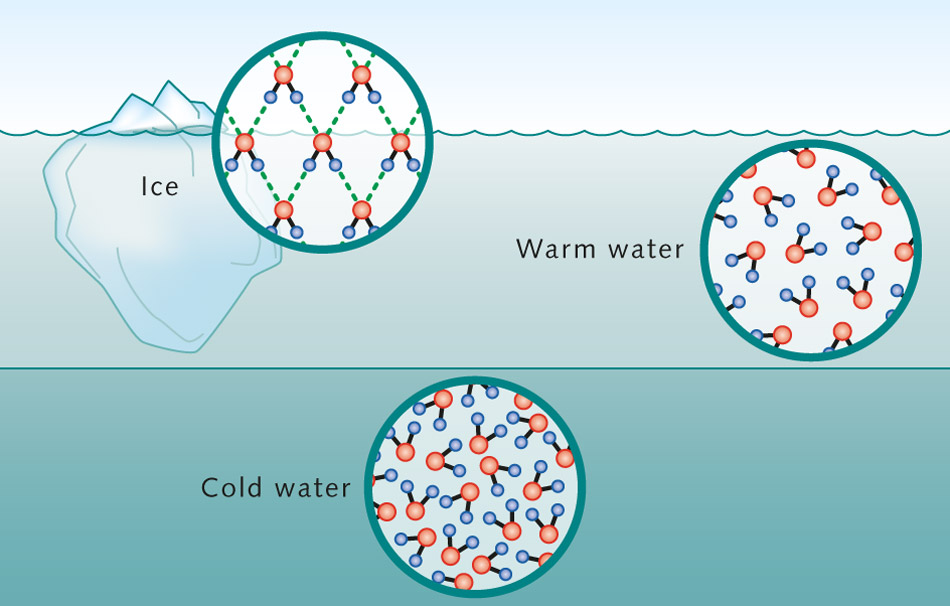
Ice, you may also notice, has the very interesting property that when you submerge it in a bath of the same material but in its liquid phase — i.e., in water — the ice doesn’t sink to the bottom, but rather rises to the top, where it floats. In its Ice Ih phase, solid water is less dense than liquid water, where approximately 10% of the ice floats above the surface of the water. If you measure the mass and volume of ice versus liquid water, you’ll find that water comes in at a density of 1.00 grams-per-cubic-centimeter, while ice has a lower density of 0.90 grams-per-cubic-centimeter.
This is incredibly important, and forms the physical basis for how ice skating can physically occur. If water/ice behaved like most materials, then simply by applying greater and greater amounts of pressure to the liquid phase would force it into a solid phase, as the atoms/molecules inside would achieve a more tightly-packed configuration.
But water is different than most materials, in that when you freeze it, it actually expands from a liquid phase into a solid phase, occupying more volume as a solid than it did as a liquid. And this means, even if you apply a tremendous amount of pressure to liquid water, it remains liquid. But if you apply that same amount of pressure to solid water (ice), it can transition into a liquid phase.
This is the key to how ice skates work! Normally, when you wear conventional shoes, sneakers, or boots, the gravitational force that pulls you down onto the Earth gets distributed over a relatively large area: the surface area of the soles of your footwear. That concept — of a force being distributed over an area — is the definition of what it means to create a pressure. If you want to raise the pressure even higher, you can either increase the amount of force that pushes down over the same area, or you can decrease the amount of area that the force is applied over.
That’s where the idea of ice skates becomes so powerful. Instead of distributing the force from your body, which can approach nearly 1000 Newtons for an average human being, over a substantial area (your footprint), they instead decrease your area tremendously: down to a long, thin “blade” of metal. If a typical skate blade is 30 centimeters long (about 1 foot) but only about 1 millimeter thick, that can create a pressure of up to three million pascals (a Newton-per-square meter) for a typical human who puts their weight down on one ice skate: about 30 times the normal amount of atmospheric pressure!
That is more than enough, when a typical skater puts their weight down on one skate (or even both skates) on the ice, for the extra, applied pressure to melt the ice beneath their skate’s blade. This causes a series of events to occur.
- First, the top layer of ice, the one in contact with their skates, turns to liquid just as their skate glides over it.
- The fact that there’s a thin, lubricating layer of liquid water between the skate above it and the solid ice beneath it reduces the skater’s friction even further.
- The liquid water, as we discussed earlier, fills in the small imperfections in the ice, creating an even smoother, lower-friction interface.
- Because there’s a decrease in the ice’s volume when it melts into the liquid phase, this creates a small “groove” in the ice for the skater to travel in.
- And finally, when the skate finishes passing over the portion of the ice that was melted, the sudden “drop” in pressure immediately causes the melted water to re-freeze, becoming ice once again.
In other words, even though we call it ice skating, the reason it’s so successful is because the skater is actually skating atop a thin layer of liquid water!

This is a temperature-dependent phenomenon. At higher temperatures, close to the freezing/melting point between ice and water, the ice is less hard, and a small increase in pressure is enough to melt the ice into a liquid phase. At lower temperatures, however, the ice is harder and quite brittle, making cracks and fissures more likely, and requiring a much greater increase in pressure to melt the ice.
For athletic events, those who maintain ice rinks leverage this to best suit the type of athletic activities that are taking place. If you’re a figure skater, you’ll generally prefer softer ice, and so an ice rink that caters to figure skating will generally keep the ice temperature at around 25 to 29 °F (-4 to -2 °C). This makes jumping and landing on the ice a slightly easier prospect than if the ice were kept colder.
On the other hand, if you value faster gliding, don’t have much use for jumping, and especially if you want an object (like a hockey puck) to move in a predictable fashion, with little sticking or bouncing, you’ll want colder, harder ice. Ice hockey rinks, as a result, are generally kept at a colder on-ice temperature of about 17 to 24 °F (-8 to -4 °C). It’s also why figure skaters and hockey players have different types of ice skates and blades: they’re optimized not only for different movements, but for different properties of ice!

If water and ice were more like the typical materials found throughout the Universe, where their solid phase is denser and has their component particles more tightly packed than their liquid phase, then ice skates would never work. Increasing the pressure over a thin region of surface would simply compress the solid, rather than melting it into a liquid phase. That’s the key property — rarely found in nature, but belonging to water and only a few other chemical compounds — that enables the high-pressures exerted by all of a human’s weight being focused down onto a narrow blade to melt, and glide over, the ice.
The fact that solids are porous and that liquids can fill in the gaps within a solid structure further helps ice skaters by reducing the friction beneath their skates, and the fact that liquid water takes up less volume than solid ice means that tiny “grooves” in the ice emerge from figure skating, providing just a tiny bit of extra stability. Finally, the fact that the water/ice phase diagram is so thoroughly pressure-dependent ensures that as soon as the skater’s blade completely passes over the location where it caused the ice to melt, that ice immediately re-freezes, leaving a solid sheet of ice for either their next pass or for the next skater.
Although for most of us, ice skating is just a simple, pleasurable way to pass the time all through the winter doldrums, there’s actually a tremendous amount of physics and chemistry at work here, all leading to your remarkable ability to glide over the ice just by wearing skates!





