New blood types are often discovered following medical disasters
- Over the last 120 years, scientists have discovered 44 blood typing systems, often following disastrous medical events.
- When a person’s immune system encounters incompatible blood, it destroys it, resulting in failed blood transfusions and pregnancy complications.
- Nowadays, physicians crossmatch blood before a transfusion.
Over the last 120 years, scientists have discovered 44 blood typing systems. These discoveries, which have saved millions of lives, often follow tragic and disastrous medical events.
A 300-year journey
In the 17th century, scientists uncovered the inner workings of the circulatory system and, before long, began injecting animal blood into people for a variety of reasons. As you might suspect, a lot of people died. By the 19th century, an English physician named James Blundell suspected animal blood wasn’t the best option. He proposed that blood is not universally compatible. That is, dogs can only tolerate dog blood, humans can only tolerate human blood, and so on.
Blundell performed blood transfusions on nine humans, and five lucky souls survived. Blundell’s success rate inspired more physicians to use human blood, but they soon discovered that mixing blood from two people would often result in clumps. Scientists suspected that patients died because these aggregates clogged up the circulatory system; however, they couldn’t figure out what caused the clumping or how to stop it.
The clumping mystery was solved at the turn of the 20th century with the emergence of a new field of biology: immunology. Early immunologists discovered that blood contains antibodies that bind and clump together foreign invaders, such as bacteria and viruses. Karl Landsteiner, a young Austrian physician, found that antibodies could do the same to foreign blood cells. Landsteiner hypothesized that there must be different types of blood cells and, through rigorous testing, developed the first blood typing system, ABO. Landsteiner received the Nobel Prize in 1930, not for developing the ABO system, but rather for discovering that blood types exist. It was a discovery that opened a new frontier in hematology research and saved millions of lives.
ABO blood group
Blood cells are coated by molecules called antigens. Some of these antigens are involved in nutrient uptake, some in communication, and others in structural integrity, but the purpose of most is unknown. The important thing to note is that antigens trigger immune responses.
The ABO blood group that Landsteiner discovered is based on two antigens, known as A and B. Type A blood cells have only antigen A, type B cells have only antigen B, type AB cells have both, and type O cells (from the German ohne, meaning “without”) have neither.
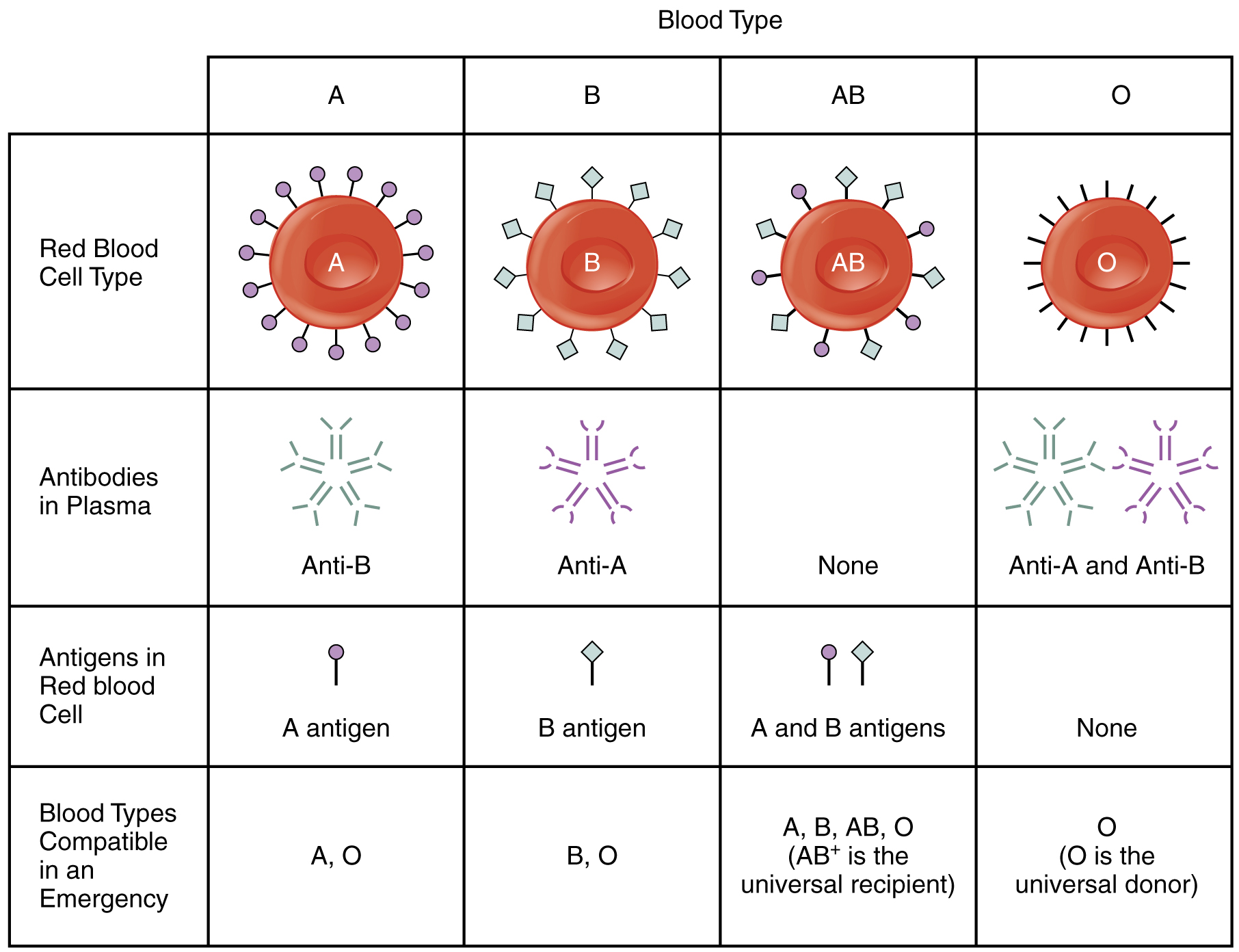
Our immune system knows which specific antigens our blood cells have and ignores them. However, if the immune system detects an unknown antigen, it will produce antibodies to attack it. For example, a person with type A blood will not produce antibodies against antigen A; however, if that person is exposed to type B or AB blood, it will produce antibodies against antigen B. This has pretty wicked consequences.
Landsteiner’s predecessors believed that blood clumps caused patients to die. However, as medical knowledge advanced, it became clear that this was only the immune system’s initial attack. After the antibodies clump the blood cells, the immune system begins destroying them, resulting in hemolytic anemia. In this way, the patients experienced massive blood loss (without actually bleeding). This is what kills most patients.
Hemolytic anemia became far less frequent following Landsteiner’s discovery, and blood transfusion quickly became a standard procedure. Physicians were careful to match patient and donor ABO blood type, but occasionally hemolytic reactions still occurred. It quickly became apparent that ABO was not the only important blood typing system.
Rh blood group
By 1932, Dr. Louis Diamond had seen a great number of newborn babies die. In fact, he had seen so many die that he noticed patterns that thousands of other physicians had missed. Four apparently distinct newborn diseases (called hydrops fetalis, erythroblastosis fetalis, icterus gravis neonatorum, and newborn anemia) were, in fact, all manifestations of the same underlying problem: a deficiency of red blood cells shortly after birth.
Unfortunately, Diamond didn’t know what caused this deficiency. He knew incompatible blood could cause hemolytic reactions; however, he ruled that out because the mother and infant often had compatible ABO blood types. The answer to Diamond’s mystery came seven years later when a heartbroken mother received a blood transfusion.
In 1939, a mother had just given birth to a stillborn child and lost a dangerous amount of blood. She and her husband were the same ABO blood type, so the physicians used his blood for the transfusion. Surprisingly, she suffered a severe hemolytic reaction as her antibodies attacked the foreign blood cells. The physicians suspected the husband’s blood possessed a rare, unknown antigen, but they could find no evidence of one. However, when they analyzed the mother’s blood, they discovered that she lacked an antigen that most people possess: the Rh antigen.
Based on Diamond’s discovery, the physicians suspected there might be a connection between the child’s death and the mother’s hemolytic reaction. They found that the baby was Rh-positive (inherited from its father), and antibodies produced by the Rh-negative mother destroyed the red blood cells of her child. Scientists soon realized that about 1 in 200 babies faced a similar reaction, killing or irreversibly harming half of them. Almost overnight, it became standard practice to test Rh blood type in addition to ABO. (Today, Rh-negative expectant mothers are given a shot to prevent them from developing anti-Rh antibodies against their baby.)
While hemolytic reactions after transfusion and during childbirth declined, they still didn’t disappear entirely. For example, scientists discovered the Vel and Langereis blood group systems after patients suffered hemolytic reactions following transfusions. Vel and Langereis antigens are far more common than even the Rh antigen. It is estimated that only 1 in 4,000 people (0.025%) lack the Val or Langereis antigen.
Diego blood group
In 1953, a child in Venezuela died of hemolytic disease three days after birth. The baby and the mother were Rh and ABO compatible, so those was ruled out. Miguel Larysse, the physician, sent the blood samples to a lab in New York that specialized in blood research. This time, they discovered that the husband possessed a rare antigen — so rare, in fact, that the testing facility could find no other person with that particular antigen. Therefore, they classified it as a “private” or “family” blood type and named it after the father’s surname, Diego.
Two years later, Mrs. Diego consulted Larysse about the risk of having another baby. Larysse combed through the Diego family history, searching for a pattern of failed pregnancies. Unsurprisingly, he found that the Diego family included ancestry from Indigenous peoples of the Americas. He wondered if the New York lab failed to find any matches because they didn’t have any blood from Indigenous peoples from South America. Indeed, studies have since found that 36% of South Americans possess the Diego antigen.
Interestingly, about 12% of Japanese and Chinese also have the antigen, and scientists have used it to map migration in Asia and the Americas. The Diego antigen is rare in Caucasians and Blacks (~0.01% are Diego-positive). However, Polish people are 50 times more likely to have the Diego antigen than other Caucasians. This is believed to be due to the invasion by the Tatars (a Turkish group of Mongolian heritage) in the 13th century.
Physicians rely on blood types less than you might think
Many people know their ABO and Rh blood type. It might be something like AB-, which is shorthand for ABO(A+B+), Rh(D-). But what about Diego, Vel, Langereis, and the other 39 blood typing systems? There is a decent chance that you lack at least one common antigen or possess one rare antigen. So what’s stopping you from having a nasty hemolytic reaction?
There are two reasons. First, many of the antigens aren’t clinically relevant. In other words, they rarely cause hemolytic reactions. For example, the Junior blood typing system is based on the expression of the Jr antigen, and people with Jr negative blood don’t always react to Jr positive blood, which is a good thing because Jr- is a very rare blood type. In a study of 9,545 Americans, not a single person lacked the antigen.
There are only ten clinically relevant blood typing systems, and if you were to expand your blood type to include them, it might look like ABO(A+B+), Rh(D+c+e+), MNS(M+N-S+s-), P1+, Lu(a+b+), Kell(K-k+), Le(a+b-), Fy(a+b-), Jk(a+b–). You don’t need to remember all this because physicians will test them before you get a transfusion.
The second reason you shouldn’t be concerned about a surprising reaction is that physicians no longer rely entirely on blood types to determine if blood is compatible. Instead, they use a technique called crossmatching, which involves mixing donors’ serum with recipients’ blood cells in a test tube. If the two are incompatible, the blood will clump. This is essentially the same technique that Landsteiner used in 1901 when he first discovered the ABO system. If it ain’t broke, don’t fix it!