Is iron the Achilles’ heel for cancer?

- Most cancer drugs cause collateral damage, disrupting the function of healthy cells.
- Some cancer cells accumulate unusually high quantities of iron.
- Iron-activated cancer drugs selectively disrupt cancer cells, while leaving healthy cells unharmed.
Good medical treatments destroy bad stuff while leaving the good stuff untouched. One of the tricky parts of treating cancer is that the stuff that needs to be destroyed (cancer cells) is painfully similar to that which needs to be untouched (healthy cells). This means drugs that kill cancer cells usually harm healthy cells as well.
In 2022, however, a team of scientists at UC San Francisco reported a way to leverage cancers’ unique metabolic profile to ensure that drugs only target cancer cells.
The collateral damage of cancer treatment
Traditional cancer treatments deploy a scorched-earth approach. For example, radiation therapy is most devastating to cells that are growing and dividing. Cancer cells are the most sensitive because they replicate a lot, but healthy cells become collateral damage. Targeted cancer treatment, on the other hand, zeroes in on molecules that play a role in how cancer cells grow and survive, such as specific receptors and enzymes. For decades, scientists have worked to identify cancer-specific molecules and drugs that block them.
For instance, MEK is an enzyme that is highly expressed in some of the most aggressive forms of pancreas, blood, and lung cancers. The overabundance of this enzyme causes a cell to divide uncontrollably. Cobimetinib, an FDA-approved cancer drug, slows the replication of cancer cells by inhibiting MEK. Unfortunately, MEK is also expressed in healthy tissues, especially the skin (where cellular turnover is rapid) and the retina (where neuronal axons are regularly regenerated). Ergo, cobimetinib also harms healthy cells.
To make matters worse, cancer cells sometimes only die when patients take relatively high doses of a drug. This is because cancer’s metabolism is often greater in cancer cells than in normal cells. For instance, some cancer cells have more MEK enzyme — meaning more cobimetinib is required to stop these cells from replicating. Unfortunately, the doses cancer patients receive often closely approach or even exceed the levels at which the drug causes toxicities in healthy tissues.
Exploiting cancer’s iron addiction
Cancer cells hoard iron at a far greater rate than healthy cells, according to previous studies. Although the reason for this remains unclear, the UCSF team realized this could be leveraged to increase the specificity of cancer drugs. If a cancer drug, such as cobimetinib, were only activated in the iron-rich environment of a cancer cell, the drug would be inert when it interacts with healthy cells. It’s something like a two-factor authentication system for cancer drugs.
To test this, the scientist synthesized an iron-activated (IA) cobimetinib that only blocks MEK in an iron-rich environment. The experimental drug inhibited tumor growth as efficiently as standard cobimetinib, but it spared healthy cells. Using a mouse-lung cancer model, mice receiving either IA-cobimetinib or standard cobimetinib had fewer lung lesions and showed prolonged overall survival compared to vehicle-treated mice. When the scientists evaluated IA-cobimetinib’s effect on healthy human retinal and skin cells, they found the healthy tissue was about 10-fold less sensitive than cancer cells to IA-cobimetinib.
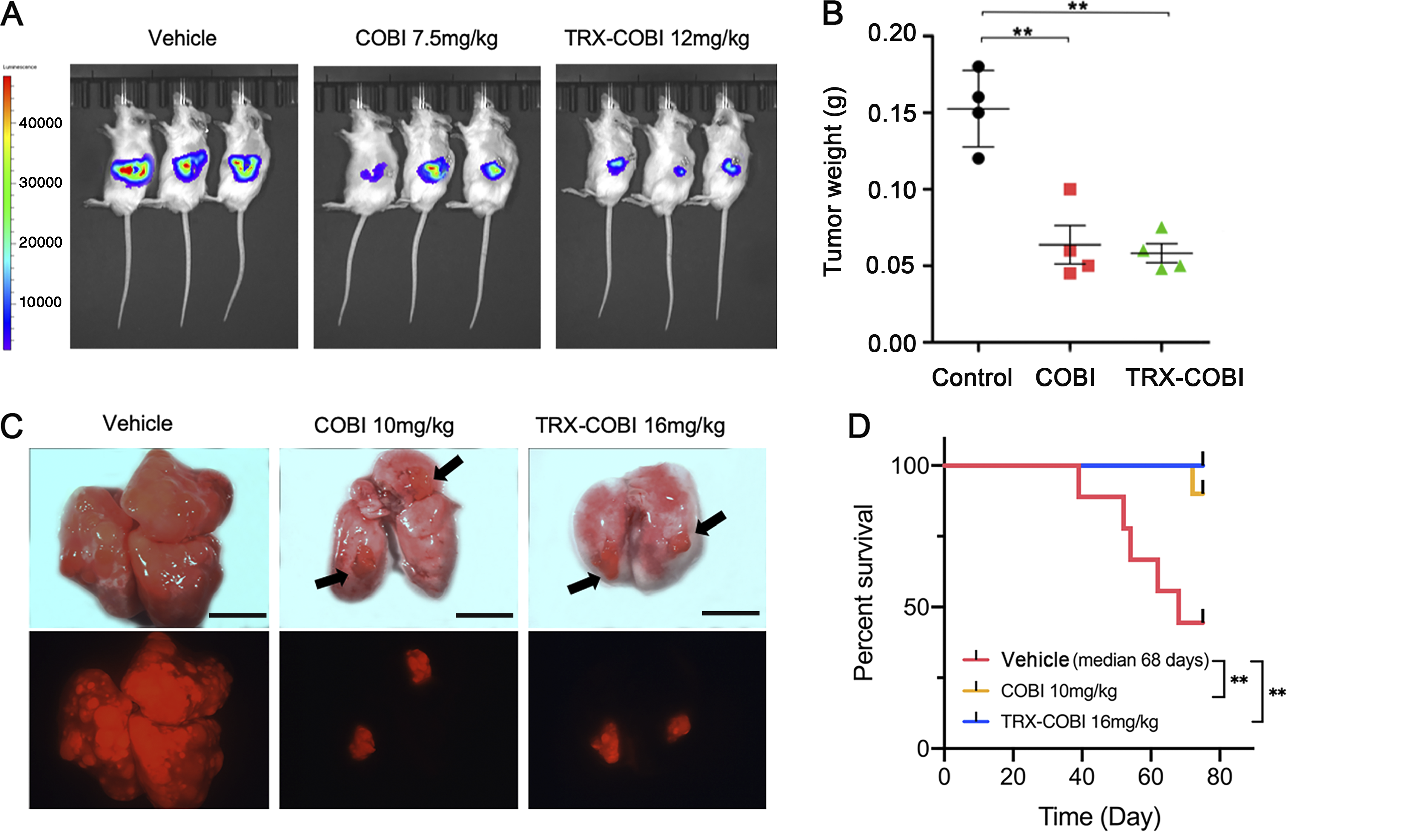
The team’s positive results have led to a commercial company licensing their iron-activated technology, according to Eric Collisson, a medical oncologist at UCSF and lead author of the study.